Health-related quality of life outcomes associated with four cisplatin-based doublet chemotherapy regimens for stage IVB recurrent or persistent cervical cancer: a Gynecologic Oncology Group study
- PMID: 20837359
- PMCID: PMC4687012
- DOI: 10.1016/j.ygyno.2010.08.020
Health-related quality of life outcomes associated with four cisplatin-based doublet chemotherapy regimens for stage IVB recurrent or persistent cervical cancer: a Gynecologic Oncology Group study
Abstract
Purpose: To assess the differences in health-related quality of life (HRQL) of 4 cisplatin containing doublet chemotherapy combinations in women with advanced/recurrent cervical carcinoma.
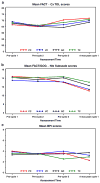
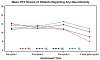
Methods: Patients were randomized to three-week cycles of paclitaxel + cisplatin (PC); vinorelbine + C (VC); gemcitabine + C (GC); or topotecan + C (TC). We report HRQL results from data available on 434 eligible patients enrolled into this 513 patient trial. HRQL was assessed with the Functional Assessment of Cancer Therapy-Cervix (FACT-Cx) the FACT/Gynecologic Oncology Group (FACT/GOG) four-item neurotoxicity scale, and the 0-10 "worst pain" item from the Brief Pain Inventory, at baseline (pre-treatment), prior to beginning cycle 2, prior to beginning cycle 5, and at 9 months after enrollment. As reported by Monk et al. (2009) [13] VC, GC and TC were found not to be superior to PC with regard to progression-free survival or overall survival.
Results: The trial was terminated early due to planned interim futility analysis, reducing power for HRQL analysis from 85% to 55%. Patients receiving VC, GC and TC doublets did not report significantly different HRQL, neuropathy, or pain from those who received the PC (control) doublet. Patients receiving PC tended to report worse neuropathy during treatment than patients who received other doublets (especially GC and TC), but the differences were not statistically significant.
Conclusion: None of the 3 experimental doublets was different from PC in terms of HRQL during treatment. Long-term toxicity data are inconclusive. Except where patients may wish to reduce their risk of worsening pre-treatment neuropathy, PC remains the standard of care for this disease.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Cecelia H. Boardman is on the Speaker’s Bureau at Glaxo-Smith-Kline, Merck and Eli Lillly. She is also a surgical proctor at Intuitive.
All other co-authors have no conflict of interest to declare.
Figures
References
-
- Cancer Facts and Figures. American Cancer Society; 2008.
-
- Long HJ, III, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626–33. - PubMed
-
- Monk BJ, Huang HQ, Cella D, Long HJ. Quality of life outcomes from a randomized phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4617–25. - PubMed
-
- Tewari KS, Monk BJ. Gynecologic oncology group trials of chemotherapy for metastatic and recurrent cervical cancer. Curr Oncol Rep. 2005;7:419–34. - PubMed
-
- Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22(15):3113–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

