Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells
- PMID: 20837470
- PMCID: PMC2975247
- DOI: 10.1074/jbc.M110.126003
Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells
Abstract
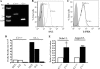
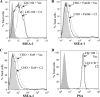
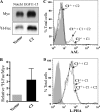
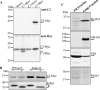
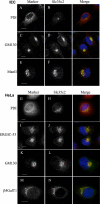
Mammalian Notch receptors require modification by fucose on epidermal growth factor-like (EGF) repeats of their extracellular domain to respond optimally to signal induction by canonical Notch ligands. Inactivation of the Golgi GDP-fucose transporter Slc35c1 in mouse or human does not cause marked defects in Notch signaling during development, and shows milder fucosylation defects than those observed in mice unable to synthesize GDP-fucose, indicating the existence of another mechanism for GDP-fucose transport into the secretory pathway. We show here that fibroblasts from mice or humans lacking Slc35c1 exhibit robust Notch signaling in co-culture signaling assays. A potential candidate for a second GDP-fucose transporter is the related gene Slc35c2. Overexpression of Slc35c2 reduces expression of the fucosylated epitopes Lewis X and sialylated Lewis X in CHO cells, indicating competition with Slc35c1. The fucosylation of a Notch1 EGF repeat fragment that occurs in the endoplasmic reticulum was increased in CHO transfectants overexpressing Slc35c2. In CHO cells with low levels of Slc35c2, both Delta1- and Jagged1-induced Notch signaling were reduced, and the fucosylation of a Notch1 fragment was also decreased. Immunofluorescence microscopy of rat intestinal epithelial cells and HeLa cells, and analysis of rat liver membrane fractions showed that Slc35c2 is primarily colocalized with markers of the cis-Golgi network and endoplasmic reticulum-Golgi intermediate compartment (ERGIC). The combined results suggest that Slc35c2 is either a GDP-fucose transporter that competes with Slc35c1 for GDP-fucose, or a factor that otherwise enhances the fucosylation of Notch and is required for optimal Notch signaling in mammalian cells.
Figures

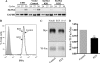

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

