Heat shock protein 90 as a drug target against protozoan infections: biochemical characterization of HSP90 from Plasmodium falciparum and Trypanosoma evansi and evaluation of its inhibitor as a candidate drug
- PMID: 20837488
- PMCID: PMC2992230
- DOI: 10.1074/jbc.M110.155317
Heat shock protein 90 as a drug target against protozoan infections: biochemical characterization of HSP90 from Plasmodium falciparum and Trypanosoma evansi and evaluation of its inhibitor as a candidate drug
Abstract
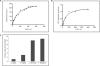
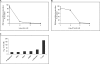
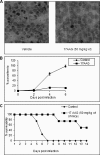
Using a pharmacological inhibitor of Hsp90 in cultured malarial parasite, we have previously implicated Plasmodium falciparum Hsp90 (PfHsp90) as a drug target against malaria. In this study, we have biochemically characterized PfHsp90 in terms of its ATPase activity and interaction with its inhibitor geldanamycin (GA) and evaluated its potential as a drug target in a preclinical mouse model of malaria. In addition, we have explored the potential of Hsp90 inhibitors as drugs for the treatment of Trypanosoma infection in animals. Our studies with full-length PfHsp90 showed it to have the highest ATPase activity of all known Hsp90s; its ATPase activity was 6 times higher than that of human Hsp90. Also, GA brought about more robust inhibition of PfHsp90 ATPase activity as compared with human Hsp90. Mass spectrometric analysis of PfHsp90 expressed in P. falciparum identified a site of acetylation that overlapped with Aha1 and p23 binding domain, suggesting its role in modulating Hsp90 multichaperone complex assembly. Indeed, treatment of P. falciparum cultures with a histone deacetylase inhibitor resulted in a partial dissociation of PfHsp90 complex. Furthermore, we found a well known, semisynthetic Hsp90 inhibitor, namely 17-(allylamino)-17-demethoxygeldanamycin, to be effective in attenuating parasite growth and prolonging survival in a mouse model of malaria. We also characterized GA binding to Hsp90 from another protozoan parasite, namely Trypanosoma evansi. We found 17-(allylamino)-17-demethoxygeldanamycin to potently inhibit T. evansi growth in a mouse model of trypanosomiasis. In all, our biochemical characterization, drug interaction, and animal studies supported Hsp90 as a drug target and its inhibitor as a potential drug against protozoan diseases.
Figures





References
-
- Walter S., Buchner J. (2002) Angew. Chem. Int. Ed. Engl. 41, 1098–1113 - PubMed
-
- Pratt W. B., Toft D. O. (2003) Exp. Biol. Med. 228, 111–133 - PubMed
-
- Wegele H., Müller L., Buchner J. (2004) Rev. Physiol. Biochem. Pharmacol. 151, 1–44 - PubMed
-
- Chiosis G., Vilenchik M., Kim J., Solit D. (2004) Drug. Discov. Today 9, 881–888 - PubMed
-
- Bagatell R., Whitesell L. (2004) Mol. Cancer Ther. 3, 1021–1030 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

