Probing electron transfer mechanisms in Shewanella oneidensis MR-1 using a nanoelectrode platform and single-cell imaging
- PMID: 20837546
- PMCID: PMC2947899
- DOI: 10.1073/pnas.1011699107
Probing electron transfer mechanisms in Shewanella oneidensis MR-1 using a nanoelectrode platform and single-cell imaging
Abstract
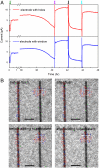
Microbial fuel cells (MFCs) represent a promising approach for sustainable energy production as they generate electricity directly from metabolism of organic substrates without the need for catalysts. However, the mechanisms of electron transfer between microbes and electrodes, which could ultimately limit power extraction, remain controversial. Here we demonstrate optically transparent nanoelectrodes as a platform to investigate extracellular electron transfer in Shewanella oneidensis MR-1, where an array of nanoholes precludes or single window allows for direct microbe-electrode contacts. Following addition of cells, short-circuit current measurements showed similar amplitude and temporal response for both electrode configurations, while in situ optical imaging demonstrates that the measured currents were uncorrelated with the cell number on the electrodes. High-resolution imaging showed the presence of thin, 4- to 5-nm diameter filaments emanating from cell bodies, although these filaments do not appear correlated with current generation. Both types of electrodes yielded similar currents at longer times in dense cell layers and exhibited a rapid drop in current upon removal of diffusible mediators. Reintroduction of the original cell-free media yielded a rapid increase in current to ∼80% of original level, whereas imaging showed that the positions of > 70% of cells remained unchanged during solution exchange. Together, these measurements show that electron transfer occurs predominantly by mediated mechanism in this model system. Last, simultaneous measurements of current and cell positions showed that cell motility and electron transfer were inversely correlated. The ability to control and image cell/electrode interactions down to the single-cell level provide a powerful approach for advancing our fundamental understanding of MFCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Fredrickson JK, et al. Towards environmental systems biology of Shewanella. Nat Rev Microbiol. 2008;6:592–603. - PubMed
-
- Bond DR, Holmes DE, Tender LM, Lovley DR. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295:483–485. - PubMed
-
- Logan BE, Regan JM. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006;14:512–518. - PubMed
-
- Lovley DR. Bug juice: Harvesting electricity with microorganisms. Nat Rev Microbiol. 2006;4:497–508. - PubMed
-
- Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol. 2009;7:375–381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

