Early impairment of transmural principal strains in the left ventricular wall after short-term, high-fat feeding of mice predisposed to cardiac steatosis
- PMID: 20837747
- PMCID: PMC3072284
- DOI: 10.1161/CIRCIMAGING.110.959098
Early impairment of transmural principal strains in the left ventricular wall after short-term, high-fat feeding of mice predisposed to cardiac steatosis
Abstract
Background: myocardial lipid accumulation precedes some cardiomyopathies, but little is known of concurrent effects on ventricular mechanics. We tested the hypothesis that intramyocardial lipid accumulation during a short-term, high-fat diet (HFD) affects 2-dimensional strains in the heart. We examined the hearts of nontransgenic (NTG) mice and of transgenic mice predisposed to elevated triacylglyceride (TAG) storage linked to low-level overexpression of peroxisome proliferator activated receptor (PPAR-α).
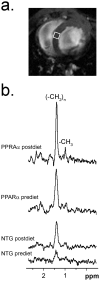
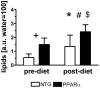
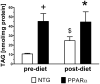
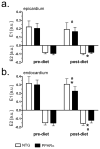
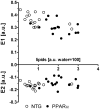
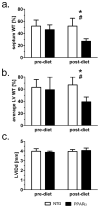
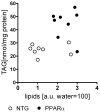
Methods and results: myocardial lipid and transmural principal strains E1 and E2 were determined in vivo with (1)H magnetic resonance spectroscopy/imaging before and after 2 weeks of an HFD in both PPAR-α and NTG littermate mice. Baseline lipid was elevated in PPAR-α compared with NTG mice. An HFD increased mobile lipid by 174% in NTG mice (P<0.05) and by 79% in PPAR-α mice (P<0.05). After an HFD, lipid and TAG were higher in PPAR-α versus NTG mice by 63% and 81%, respectively. However, TAG in PPAR-α mice after an HFD was similar to TAG in PPAR-α mice fed a regular diet, suggesting that the magnetic resonance spectroscopy signal from lipid is not exclusive to TAG. Only at the highest lipid contents, achieved in PPAR-α mice, were strains affected. Endocardial strain was most compromised, with a negative correlation to lipid (P<0.05).
Conclusions: a short-term HFD elevated myocardial lipid measures as determined by magnetic resonance spectroscopy, which became dissociated from TAG content in hearts predisposed to cardiac steatosis. The increased lipid was associated with concurrent, transmural reductions in E1 and E2 strains across the left ventricular wall. Strains were attenuated at the highest levels of lipid accumulation, suggesting a threshold response. Thus, 2-dimensional strains are impaired early and without left ventricular diastolic dysfunction, owing to cardiac steatosis.
Figures
References
-
- McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac Steatosis in Diabetes Mellitus. A 1H-magnetc Resonance Spectroscopy Study. Circulation. 2007;116:1170–1175. - PubMed
-
- Schaffer JE. Lipotoxicity: when tissue overeat. Curr Opin Lipidol. 2003;14:281–287. - PubMed
-
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmayer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. - PubMed
-
- Weinberg JM. Lipotoxicity. Kindney International. 2006;70:1460–1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

