Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production
- PMID: 20837772
- PMCID: PMC3101588
- DOI: 10.1083/jcb.201002111
Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production
Abstract
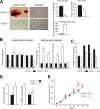
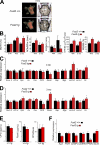
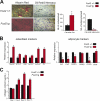
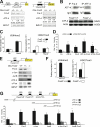
The activator protein-1 (AP-1) transcription factor complex, in particular the Fos proteins, is an important regulator of bone homeostasis. Fra-2 (Fosl2), a Fos-related protein of the AP-1 family, is expressed in bone cells, and newborn mice lacking Fra-2 exhibit defects in chondrocytes and osteoclasts. Here we show that Fra-2-deficient osteoblasts display a differentiation defect both in vivo and in vitro. Moreover, Fra-2-overexpressing mice are osteosclerotic because of increased differentiation of osteoblasts, which appears to be cell autonomous. Importantly, the osteoblast-specific osteocalcin (Oc) gene and collagen1α2 (col1α2) are transcriptional targets of Fra-2 in both murine and human bone cells. In addition, Fra-2, Oc, and col1 are expressed in stromal cells of human chondroblastic and osteoblastic osteosarcomas (Os's) as well as during osteoblast differentiation of human Os cell lines. These findings reveal a novel function of Fra-2/AP-1 as a positive regulator of bone and matrix formation in mice and humans.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

