Association between pharmaceutical support and basic science research on erythropoiesis-stimulating agents
- PMID: 20837837
- PMCID: PMC4138541
- DOI: 10.1001/archinternmed.2010.309
Association between pharmaceutical support and basic science research on erythropoiesis-stimulating agents
Abstract
Background: To our knowledge, no prior research has evaluated the association between pharmaceutical industry funding and basic science research results. When erythropoiesis-stimulating agents (ESAs) were licensed to treat chemotherapy-associated anemia, basic science concerns related to potential cancer stimulation were raised. We evaluated associations between pharmaceutical industry support and reported findings evaluating ESA effects on cancer cells.
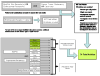
Methods: Articles identified in MEDLINE and EMBASE databases (1988-2008) investigating basic science findings related to ESA administration in the solid tumor setting were reviewed. Outcomes included information on erythropoietin receptors (EpoRs), Epo-induced signaling events, cellular function, and qualitative conclusions. Information on study funding (academic investigators with no reported funding from ESA manufacturers [64 studies], academic investigators with grant funding from ESA manufacturers [7 studies], and investigators employed by the ESA manufacturers [3 studies]) was evaluated. Some studies did not include information on each outcome.
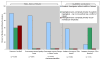
Results: Investigators without funding from ESA manufacturers were more likely than academic investigators with such funding or investigators employed by ESA manufacturers to identify EpoRs on solid tumor cells (100%, 60%, and 67%, respectively; P = .009), Epo-induced signaling events (94%, 0%, and 0%, respectively; P = .001), or changes in cellular function (57%, 0%, and 0%, respectively; P = .007) and to conclude that ESAs had potentially harmful effects on cancer cells (57%, 0%, and 0%, respectively; P = .008).
Conclusions: Researchers who do not have pharmaceutical industry support are more likely than those with pharmaceutical support to identify detrimental in vitro effects of ESAs. The potential for conflicts of interest to affect basic science research should be considered.
Conflict of interest statement
Charles L. Bennett MD PhD MPP: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Simone N. Boyle BA: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Adam Kuykendal MD: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Matthew J. Fisher BA: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Athena T. Samaras BA: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Sara E. Barnato MD: I declare that I participated in the writing and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Robin L. Wagner BS: I declare that I participated in the literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Carolyn E. Goldstein BA: I declare that I participated in the literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Jacob Tallman: I declare that I participated in the literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Hidayatullah G. Munshi MD: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Stephen Y. Lai MD PhD: I declare that I participated in the planning, writing, and literature review for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Michael Henke MD: I declare that I participated in the planning and writing for this paper and that I have seen and approved the final version. I have no conflicts of interests to disclose.
Figures

References
-
- Kaiser J. Conflicts of interest. Cardiologists come under the glare of a Senate inquiry. Science. 2008;322(5901):513. - PubMed
-
- Lurie P, Almeida CM, Stine N, Stine AR, Wolfe SM. Financial conflict of interest disclosure and voting patterns at Food and Drug Administration Drug Advisory Committee meetings. Jama. 2006;295(16):1921–8. - PubMed
-
- Stelfox HT, Chua G, O’Rourke K, Detsky AS. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med. 1998;338(2):101–6. - PubMed
-
- Friedberg M, Saffran B, Stinson TJ, Nelson W, Bennett CL. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. Jama. 1999;282(15):1453–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

