Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: subpopulation treatment effect pattern plot
- PMID: 20837942
- PMCID: PMC2988642
- DOI: 10.1200/JCO.2009.27.9182
Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: subpopulation treatment effect pattern plot
Abstract
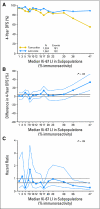
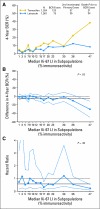
The discovery of biomarkers that predict treatment effectiveness has great potential for improving medical care, particularly in oncology. These biomarkers are increasingly reported on a continuous scale, allowing investigators to explore how treatment efficacy varies as the biomarker values continuously increase, as opposed to using arbitrary categories of expression levels resulting in a loss of information. In the age of biomarkers as continuous predictors (eg, expression level percentage rather than positive v negative), alternatives to such dichotomized analyses are needed. The purpose of this article is to provide an overview of an intuitive statistical approach-the subpopulation treatment effect pattern plot (STEPP)-for evaluating treatment-effect heterogeneity when a biomarker is measured on a continuous scale. STEPP graphically explores the patterns of treatment effect across overlapping intervals of the biomarker values. As an example, STEPP methodology is used to explore patterns of treatment effect for varying levels of the biomarker Ki-67 in the BIG (Breast International Group) 1-98 randomized clinical trial comparing letrozole with tamoxifen as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer. STEPP analyses showed patients with higher Ki-67 values who were assigned to receive tamoxifen had the poorest prognosis and may benefit most from letrozole.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Yeatman TJ. Predictive biomarkers: Identification and verification. J Clin Oncol. 2009;27:2743–2744. - PubMed
-
- Cox DR. Regression models and life tables (with discussion) J Roy Stat Soc B. 1972;34:187–220.
-
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
-
- Royston P, Altman D, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat Med. 2006;25:127–141. - PubMed
-
- Bonetti M, Gelber RD. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med. 2000;19:2595–2609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

