Advances in understanding tissue regenerative capacity and mechanisms in animals
- PMID: 20838411
- PMCID: PMC3069856
- DOI: 10.1038/nrg2879
Advances in understanding tissue regenerative capacity and mechanisms in animals
Abstract
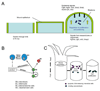
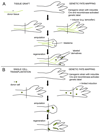
Questions about how and why tissue regeneration occurs have captured the attention of countless biologists, biomedical engineers and clinicians. Regenerative capacity differs greatly across organs and organisms, and a range of model systems that use different regenerative strategies and that offer different technical advantages have been studied to understand regeneration. Making use of this range of systems and approaches, recent advances have allowed progress to be made in understanding several key issues that are common to natural regenerative events. These issues include: the determination of regenerative capacity; the importance of stem cells, dedifferentiation and transdifferentiation; how regenerative signals are initiated and targeted; and the mechanisms that control regenerative proliferation and patterning.
Figures



References
-
- Morgan TH. Experimental studies of the regeneration of Planaria maculata. Arch. Entw. Mech. Org. 1898;7:364–397.
-
- Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. - PubMed
-
- Gierer A, et al. Regeneration of hydra from reaggregated cells. Nat New Biol. 1972;239:98–101. - PubMed
-
- Bosch TC. Why polyps regenerate and we don't: towards a cellular and molecular framework for Hydra regeneration. Dev Biol. 2007;303:421–433. - PubMed
-
- Garza-Garcia AA, Driscoll PC, Brockes JP. Evidence for the local evolution of mechanisms underlying limb regeneration in salamanders. Integrative and Comparative Biology. 2010:1–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

