Regulation of inflammatory gene expression in PBMCs by immunostimulatory botanicals
- PMID: 20838436
- PMCID: PMC2933230
- DOI: 10.1371/journal.pone.0012561
Regulation of inflammatory gene expression in PBMCs by immunostimulatory botanicals
Abstract
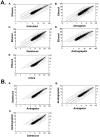
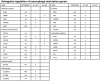
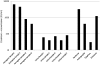
Many hundreds of botanicals are used in complementary and alternative medicine for therapeutic use as antimicrobials and immune stimulators. While there exists many centuries of anecdotal evidence and few clinical studies on the activity and efficacy of these botanicals, limited scientific evidence exists on the ability of these botanicals to modulate the immune and inflammatory responses. Using botanogenomics (or herbogenomics), this study provides novel insight into inflammatory genes which are induced in peripheral blood mononuclear cells following treatment with immunomodulatory botanical extracts. These results may suggest putative genes involved in the physiological responses thought to occur following administration of these botanical extracts. Using extracts from immunostimulatory herbs (Astragalus membranaceus, Sambucus cerulea, Andrographis paniculata) and an immunosuppressive herb (Urtica dioica), the data presented supports previous cytokine studies on these herbs as well as identifying additional genes which may be involved in immune cell activation and migration and various inflammatory responses, including wound healing, angiogenesis, and blood pressure modulation. Additionally, we report the presence of lipopolysaccharide in medicinally prepared extracts of these herbs which is theorized to be a natural and active component of the immunostimulatory herbal extracts. The data presented provides a more extensive picture on how these herbs may be mediating their biological effects on the immune and inflammatory responses.
Conflict of interest statement
Figures




Similar articles
-
The Role of Endophytic/Epiphytic Bacterial Constituents in the Immunostimulatory Activity of the Botanical, Astragalus membranaceus.Yale J Biol Med. 2020 Jun 29;93(2):239-250. eCollection 2020 Jun. Yale J Biol Med. 2020. PMID: 32607085 Free PMC article.
-
Multiple modulatory activities of Andrographis paniculata on immune responses and xenograft growth in esophageal cancer preclinical models.Phytomedicine. 2019 Jul;60:152886. doi: 10.1016/j.phymed.2019.152886. Epub 2019 Mar 12. Phytomedicine. 2019. PMID: 30910259
-
Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds.Phytomedicine. 2014 Jan 15;21(2):164-71. doi: 10.1016/j.phymed.2013.08.008. Epub 2013 Sep 14. Phytomedicine. 2014. PMID: 24041614 Review.
-
Screening of immuno-modulatory potential of different herbal plant extracts using striped catfish (Pangasianodon hypophthalmus) leukocyte-based in vitro tests.Fish Shellfish Immunol. 2019 Oct;93:296-307. doi: 10.1016/j.fsi.2019.07.064. Epub 2019 Jul 25. Fish Shellfish Immunol. 2019. PMID: 31352112
-
Screening of pharmacological uses of Urtica dioica and others benefits.Prog Biophys Mol Biol. 2020 Jan;150:67-77. doi: 10.1016/j.pbiomolbio.2019.05.008. Epub 2019 Jun 1. Prog Biophys Mol Biol. 2020. PMID: 31163183 Review.
Cited by
-
Immunomodulatory effect of a proprietary polyherbal formulation on healthy participants: A single- blind, randomized, placebo- controlled, exploratory clinical study.Perspect Clin Res. 2023 Jul-Sep;14(3):130-138. doi: 10.4103/picr.picr_100_22. Epub 2023 May 22. Perspect Clin Res. 2023. PMID: 37554241 Free PMC article.
-
Astragalus root and elderberry fruit extracts enhance the IFN-β stimulatory effects of Lactobacillus acidophilus in murine-derived dendritic cells.PLoS One. 2012;7(10):e47878. doi: 10.1371/journal.pone.0047878. Epub 2012 Oct 30. PLoS One. 2012. PMID: 23118903 Free PMC article.
-
Studies on the mitochondrial, immunological and inflammatory effects of solvent fractions of Diospyros mespiliformis Hochst in Plasmodium berghei-infected mice.Sci Rep. 2021 Mar 25;11(1):6941. doi: 10.1038/s41598-021-85790-6. Sci Rep. 2021. PMID: 33767260 Free PMC article.
-
Oral Chinese herbal medicine combined with pharmacotherapy for stable COPD: a systematic review of effect on BODE index and six minute walk test.PLoS One. 2014 Mar 12;9(3):e91830. doi: 10.1371/journal.pone.0091830. eCollection 2014. PLoS One. 2014. PMID: 24622390 Free PMC article.
-
A crossover randomized controlled trial examining the effects of black seed (Nigella sativa) supplementation on IL-1β, IL-6 and leptin, and insulin parameters in overweight and obese women.BMC Complement Med Ther. 2024 Jan 5;24(1):22. doi: 10.1186/s12906-023-04226-y. BMC Complement Med Ther. 2024. PMID: 38178093 Free PMC article. Clinical Trial.
References
-
- Astin J. Why patients use alternative medicine: results of a national study. JAMA. 1998;279:1548–1553. - PubMed
-
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. - PubMed
-
- Ritchie Use of herbal supplements and nutritional supplements in the UK: what do we know about their pattern of usage? Proc Nutr Soc. 2007;66:479–482. - PubMed
-
- Fu PP, Chiang HM, Xia Q, Chen T, Chen BH, et al. Quality assurance and safety of herbal dietary supplements. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:91–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

