Rewiring neural interactions by micro-stimulation
- PMID: 20838477
- PMCID: PMC2936935
- DOI: 10.3389/fnsys.2010.00039
Rewiring neural interactions by micro-stimulation
Abstract
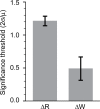
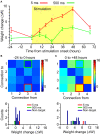
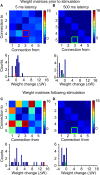
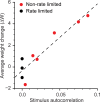
Plasticity is a crucial component of normal brain function and a critical mechanism for recovery from injury. In vitro, associative pairing of presynaptic spiking and stimulus-induced postsynaptic depolarization causes changes in the synaptic efficacy of the presynaptic neuron, when activated by extrinsic stimulation. In vivo, such paradigms can alter the responses of whole groups of neurons to stimulation. Here, we used in vivo spike-triggered stimulation to drive plastic changes in rat forelimb sensorimotor cortex, which we monitored using a statistical measure of functional connectivity inferred from the spiking statistics of the neurons during normal, spontaneous behavior. These induced plastic changes in inferred functional connectivity depended on the latency between trigger spike and stimulation, and appear to reflect a robust reorganization of the network. Such targeted connectivity changes might provide a tool for rerouting the flow of information through a network, with implications for both rehabilitation and brain-machine interface applications.
Keywords: brain machine interface; functional connectivity; hebbian association; plasticity; rat; sensorimotor cortex.
Figures






References
-
- Baccala L. A., Sameshima K. (2001). “Overcoming the limitations of correlation analysis for many simultaneously processed neural structures,” in Progress in Brain Research, Vol. 130 ed. Nicolelis M. A. (New York: Elsevier; ), 33–47 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

