Preparation of Coated Valproic Acid and Sodium Valproate Sustained-release Matrix Tablets
- PMID: 20838520
- PMCID: PMC2929775
- DOI: 10.4103/0250-474X.65026
Preparation of Coated Valproic Acid and Sodium Valproate Sustained-release Matrix Tablets
Abstract
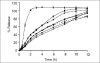
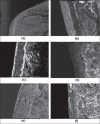
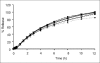
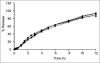
The aim of this research was to investigate the technique for preparation of coated valproic acid and sodium valproate sustained-release matrix tablets. Different diluents were tested and selected as the effective absorbent for oily valproic acid. Effect of the amount of absorbent and hydroxypropylmethylcellulose on drug release from valproic acid-sodium valproate matrix tablets prepared with wet granulation technique was evaluated in pH change system. Colloidal silicon dioxide effectively adsorbed liquid valproic acid during wet granulation and granule preparation. The amounts of colloidal silicon dioxide and hydroxypropylmethylcellulose employed in tablet formulations affected drug release from the tablets. The drug release was prominently sustained for over 12 h using hydroxypropylmethylcellulose-based hydrophilic matrix system. The mechanism of drug release through the matrix polymer was a diffusion control. The drug release profile of the developed matrix tablet was similar to Depakine Chrono(®), providing the values of similarity factor (f2) and difference factor (f1) of 85.56 and 2.37, respectively. Eudragit(®) L 30 D-55 was used as effective subcoating material for core matrix tablets before over coating with hydroxypropylmethylcellulose film with organic base solvent. Drug release profile of coated matrix tablet was almost similar to that of Depakine Chrono(®).
Keywords: Coated matrix; preparation technique; sodium valproate; sustained-release; valproic acid.
Figures



Similar articles
-
Design and optimization of sustained-release divalproex sodium tablets with response surface methodology.AAPS PharmSciTech. 2013 Mar;14(1):245-53. doi: 10.1208/s12249-012-9907-z. Epub 2012 Dec 27. AAPS PharmSciTech. 2013. PMID: 23269542 Free PMC article.
-
A Three-Pulse Release Tablet for Amoxicillin: Preparation, Pharmacokinetic Study and Physiologically Based Pharmacokinetic Modeling.PLoS One. 2016 Aug 1;11(8):e0160260. doi: 10.1371/journal.pone.0160260. eCollection 2016. PLoS One. 2016. PMID: 27479702 Free PMC article.
-
Formulation Development and Optimization of Matrix Tablet of Tramadol Hydrochloride.Recent Pat Drug Deliv Formul. 2017;11(1):19-27. doi: 10.2174/1872211310666161004160304. Recent Pat Drug Deliv Formul. 2017. PMID: 27712568
-
Subcoating with Kollidon VA 64 as water barrier in a new combined native dextran/HPMC-cetyl alcohol controlled release tablet.Eur J Pharm Biopharm. 2008 May;69(1):303-11. doi: 10.1016/j.ejpb.2007.10.012. Epub 2007 Oct 30. Eur J Pharm Biopharm. 2008. PMID: 18053697
-
Influence of polymeric subcoats on the drug release properties of tablets powder-coated with pre-plasticized Eudragit L 100-55.Int J Pharm. 2009 Feb 9;367(1-2):20-8. doi: 10.1016/j.ijpharm.2008.09.020. Epub 2008 Sep 20. Int J Pharm. 2009. PMID: 18848872
Cited by
-
Preparation, optimization and in vitro-in vivo evaluation of Shunxin sustained release granules.Chin Med. 2019 Sep 23;14:36. doi: 10.1186/s13020-019-0255-8. eCollection 2019. Chin Med. 2019. PMID: 31572488 Free PMC article.
-
Development and Characterization of Hygroscopicity-Controlled Sustain Release Formulation of Divalproex Sodium.Turk J Pharm Sci. 2022 Aug 31;19(4):422-430. doi: 10.4274/tjps.galenos.2021.57615. Turk J Pharm Sci. 2022. PMID: 36047573 Free PMC article.
-
Design and optimization of sustained-release divalproex sodium tablets with response surface methodology.AAPS PharmSciTech. 2013 Mar;14(1):245-53. doi: 10.1208/s12249-012-9907-z. Epub 2012 Dec 27. AAPS PharmSciTech. 2013. PMID: 23269542 Free PMC article.
-
Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia.Pharmaceutics. 2020 Sep 11;12(9):866. doi: 10.3390/pharmaceutics12090866. Pharmaceutics. 2020. PMID: 32933001 Free PMC article.
References
-
- Conti S, Maggi L, Segale L, Machiste E, Conte U, Grenier P, et al. Matrices containing NaCMC and HPMC 2: Swelling and release mechanism study. Int J Pharm. 2007;333:143–51. - PubMed
-
- Hardy IJ, Cook WG, Melia CD. Compression and compaction properties of plasticised high molecular weight hydroxypropylmethylcellulose (HPMC) as a hydrophilic matrix carrier. Int J Pharm. 2006;311:26–32. - PubMed
-
- Jonat S, Albers P, Gray A, Schmidt PC. Investigation of the glidant properties of compacted colloidal silicon dioxide by angle of repose and X-ray photoelectron spectroscopy. Eur J Pharm Biopharm. 2006;63:356–9. - PubMed
-
- Albertini B, Passerini N, Gonzalez-Rodriguez ML, Perissutti B, Rodriguez L. Effect of Aerosil® on the properties of lipid controlled release microparticles. J Control Release. 2004;100:233–46. - PubMed
-
- Chang ZL. Sodium valproate and valproic acid. In: Florey K, editor. Analytical Profile of Drug Substance. Vol. 8. New York: Academic Press; 1979. pp. 529–54.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
