Incremental genetic perturbations to MCM2-7 expression and subcellular distribution reveal exquisite sensitivity of mice to DNA replication stress
- PMID: 20838603
- PMCID: PMC2936539
- DOI: 10.1371/journal.pgen.1001110
Incremental genetic perturbations to MCM2-7 expression and subcellular distribution reveal exquisite sensitivity of mice to DNA replication stress
Abstract
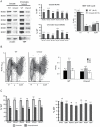
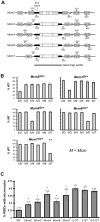
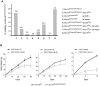
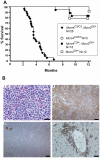
Mutations causing replication stress can lead to genomic instability (GIN). In vitro studies have shown that drastic depletion of the MCM2-7 DNA replication licensing factors, which form the replicative helicase, can cause GIN and cell proliferation defects that are exacerbated under conditions of replication stress. To explore the effects of incrementally attenuated replication licensing in whole animals, we generated and analyzed the phenotypes of mice that were hemizygous for Mcm2, 3, 4, 6, and 7 null alleles, combinations thereof, and also in conjunction with the hypomorphic Mcm4(Chaos3) cancer susceptibility allele. Mcm4(Chaos3/Chaos3) embryonic fibroblasts have ∼40% reduction in all MCM proteins, coincident with reduced Mcm2-7 mRNA. Further genetic reductions of Mcm2, 6, or 7 in this background caused various phenotypes including synthetic lethality, growth retardation, decreased cellular proliferation, GIN, and early onset cancer. Remarkably, heterozygosity for Mcm3 rescued many of these defects. Consistent with a role in MCM nuclear export possessed by the yeast Mcm3 ortholog, the phenotypic rescues correlated with increased chromatin-bound MCMs, and also higher levels of nuclear MCM2 during S phase. The genetic, molecular and phenotypic data demonstrate that relatively minor quantitative alterations of MCM expression, homeostasis or subcellular distribution can have diverse and serious consequences upon development and confer cancer susceptibility. The results support the notion that the normally high levels of MCMs in cells are needed not only for activating the basal set of replication origins, but also "backup" origins that are recruited in times of replication stress to ensure complete replication of the genome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

