Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance
- PMID: 20838642
- PMCID: PMC2935388
- DOI: 10.1371/journal.pone.0012618
Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance
Abstract
Background: Intermittent Preventive Treatment of malaria in infants using sulfadoxine-pyrimethamine (SP-IPTi) is recommended by WHO for implementation in settings where resistance to SP is not high. Here we examine the relationship between the protective efficacy of SP-IPTi and measures of SP resistance.
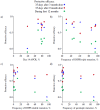
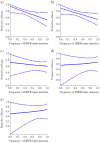
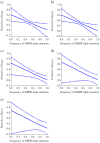
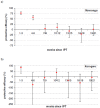
Methods and results: We analysed the relationship between protective efficacy reported in the 7 SP-IPTi trials and contemporaneous data from 6 in vivo efficacy studies using SP and 7 molecular studies reporting frequency of dhfr triple and dhps double mutations within 50 km of the trial sites. We found a borderline significant association between frequency of the dhfr triple mutation and protective efficacy to 12 months of age of SP-IPTi. This association is significantly biased due to differences between studies, namely number of doses of SP given and follow up times. However, fitting a simple probabilistic model to determine the relationship between the frequency of the dhfr triple, dhps double and dhfr/dhps quintuple mutations associated with resistance to SP and protective efficacy, we found a significant inverse relationship between the dhfr triple mutation frequency alone and the dhfr/dhps quintuple mutations and efficacy at 35 days post the 9 month dose and up to 12 months of age respectively.
Conclusions: A significant relationship was found between the frequency of the dhfr triple mutation and SP-IPTi protective efficacy at 35 days post the 9 month dose. An association between the protective efficacy to 12 months of age and dhfr triple and dhfr/dhps quintuple mutations was found but should be viewed with caution due to bias. It was not possible to define a more definite relationship based on the data available from these trials.
Conflict of interest statement
Figures


References
-
- Kobbe R, Kreuzberg C, Adjei S, Thompson B, Langefeld I, et al. A randomized controlled trial of extended intermittent preventive antimalarial treatment in infants. Clin Infect Dis. 2007;45:16–25. - PubMed
-
- Macete E, Aide P, Aponte JJ, Sanz S, Mandomando I, et al. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J Infect Dis. 2006;194:276–285. - PubMed
-
- Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001;357:1471–1477. - PubMed

