Drosophila Chk2 and p53 proteins induce stage-specific cell death independently during oogenesis
- PMID: 20838898
- PMCID: PMC3047510
- DOI: 10.1007/s10495-010-0539-z
Drosophila Chk2 and p53 proteins induce stage-specific cell death independently during oogenesis
Abstract
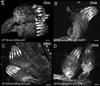
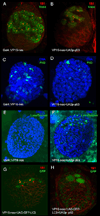
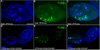
In Drosophila, the checkpoint protein-2 kinase (DmChk2) and its downstream effector protein, Dmp53, are required for DNA damage-mediated cell cycle arrest, DNA repair and apoptosis. In this study we focus on understanding the function of these two apoptosis inducing factors during ovarian development. We found that expression of Dmp53, but not DmChk2, led to loss of ovarian stem cells. We demonstrate that expression of DmChk2, but not Dmp53, induced mid-oogenesis cell death. DmChk2 induced cell death was not suppressed by Dmp53 mutant, revealing for the first time that in Drosophila, over-expression of DmChk2 can induce cell death which is independent of Dmp53. We found that over-expression of caspase inhibitors such as DIAP1, p35 and p49 did not suppress DmChk2- and Dmp53-induced cell death. Thus, our study reveals stage-specific effects of Dmp53 and DmChk2 in oogenesis. Moreover, our results demonstrate that although DmChk2 and Dmp53 affect different stages of ovarian development, loss of ovarian stem cells by p53 expression and mid-oogenesis cell death induced by DmChk2 do not require caspase activity.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

