Saposin C coupled lipid nanovesicles enable cancer-selective optical and magnetic resonance imaging
- PMID: 20838909
- PMCID: PMC4627685
- DOI: 10.1007/s11307-010-0417-7
Saposin C coupled lipid nanovesicles enable cancer-selective optical and magnetic resonance imaging
Abstract
Purpose: Nanovesicles composed of the phospholipid dioleylphosphatidylserine (DOPS) and a fusogenic protein, saposin C (SapC), selectively target and induce apoptotic cell death in a variety of human cancer cells in vitro and in vivo. We tested whether such tumor-homing nanovesicles are capable of delivering fluorescent probes and magnetic resonance (MR) contrast agents to cancerous tissue to aid in earlier detection and improve visualization.
Procedures: SapC-DOPS nanovesicles labeled with either a far-red fluorescent probe (CellVue® Maroon, CVM) or conjugated with a dextran coated MR contrast agent, ultrasmall superparamagnetic iron oxide (USPIO), were systemically administrated into xenografts for tumor detection using optical and MR imaging systems.
Results: SapC-DOPS nanovesicles were effectively detected in vivo in tumor-bearing animals using both optical and MR imaging techniques, thereby demonstrating the cancer-selective properties of these nanovesicles.
Conclusions: SapC-DOPS nanovesicles offer promise as a new and robust theranostic agent for broad cancer-selective detection, visualization, and potential therapy.
Figures
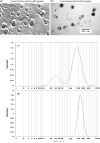




References
-
- Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nature Protoc. 2006;1:775–782. - PubMed
-
- Marx V. Molecular imaging: companies set out to sharpen the in vivo perspective with new machines and novel contrast agents. Chem Eng News. 2005;83:25–34.
-
- Fernandes RS, Kirszberg C, Rumjanek VM, et al. On the molecular mechanisms for the highly procoagulant pattern of C6 glioma cells. J Thromb Haemost. 2006;4:1546–52. - PubMed
-
- Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–40. - PubMed
-
- Ran S, Thorpe PE. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys. 2002;54:1479–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

