Stereoselective C-glycosidations with achiral and enantioenriched allenylsilanes
- PMID: 20839812
- PMCID: PMC3156056
- DOI: 10.1021/ol1019629
Stereoselective C-glycosidations with achiral and enantioenriched allenylsilanes
Erratum in
- Org Lett. 2010 Dec 3;12(23):5600
Abstract
Allenylsilanes are used as carbon nucleophiles in highly stereoselective Lewis acid-promoted C-glycosidations, resulting in the introduction of an internal alkyne with an adjacent stereocenter. Both achiral and chiral allenylsilanes form the desired products with high diastereoselectivity, where the nucleophile adds exclusively to the α-face of the intermediate oxonium ion. Reactions with glucal and galactal afford dihydropyran products, while reactions with a ribose derivative yield dihydrofuran products.
Figures

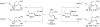
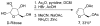

Similar articles
-
Preparation and use of enantioenriched allenylsilanes for the stereoselective synthesis of homopropargylic ethers.Org Lett. 2007 Jul 5;9(14):2689-92. doi: 10.1021/ol070936d. Epub 2007 Jun 9. Org Lett. 2007. PMID: 17559219
-
Regioselective prins cyclization of allenylsilanes. Stereoselective formation of multisubstituted heterocyclic compounds.Org Lett. 2012 Dec 21;14(24):6130-3. doi: 10.1021/ol302669q. Epub 2012 Dec 13. Org Lett. 2012. PMID: 23237587
-
Synthesis of enantioenriched homopropargylic sulfonamides by a three component reaction of aldehydes, sulfonamides, and chiral allenylsilanes.Org Lett. 2009 Oct 1;11(19):4362-5. doi: 10.1021/ol901692n. Org Lett. 2009. PMID: 19775184 Free PMC article.
-
Annulations of enantioenriched allenylsilanes with in situ generated iminium ions: stereoselective synthesis of diverse heterocycles.Org Lett. 2009 Jan 15;11(2):473-6. doi: 10.1021/ol802618p. Org Lett. 2009. PMID: 19072097 Free PMC article.
-
Enantioselective catalysis and complexity generation from allenoates.Chem Soc Rev. 2009 Nov;38(11):3102-16. doi: 10.1039/b816700c. Epub 2009 Jul 24. Chem Soc Rev. 2009. PMID: 19847345 Review.
Cited by
-
Highly Stereoselective Glycosylation Reactions of Furanoside Derivatives via Rhenium (V) Catalysis.J Org Chem. 2021 Jun 4;86(11):7672-7686. doi: 10.1021/acs.joc.1c00706. Epub 2021 May 25. J Org Chem. 2021. PMID: 34033490 Free PMC article.
-
Efficient carbon-Ferrier rearrangement on glycals mediated by ceric ammonium nitrate: Application to the synthesis of 2-deoxy-2-amino-C-glycoside.Beilstein J Org Chem. 2014 Jan 30;10:300-6. doi: 10.3762/bjoc.10.27. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24605151 Free PMC article.
-
Generation of Axially Chiral Fluoroallenes through a Copper-Catalyzed Enantioselective β-Fluoride Elimination.J Am Chem Soc. 2021 Sep 1;143(34):13759-13768. doi: 10.1021/jacs.1c05769. Epub 2021 Aug 16. J Am Chem Soc. 2021. PMID: 34465099 Free PMC article.
References
-
- Ferrier RJ. Top Curr Chem. 2001;215:153–175.
-
- Lewis MD, Cha JK, Kishi Y. J Am Chem Soc. 1982;104:4976–4978.
- Panek JS, Sparks MA. J Org Chem. 1989;54:2034–2038.
- Larsen CH, Ridgway BH, Shaw JT, Woerpel KA. J Am Chem Soc. 1999;121:12208–12209.
- Romero JAC, Tobacco SA, Woerpel KA. J Am Chem Soc. 2000;122:168–169.
-
- Danishefsky SJ, Kerwin JF. J Org Chem. 1982;47:3803–3805.
- Danishefksy SJ, Armistead DM, Wincott FE, Selnick HG, Hungate R. J Am Chem Soc. 1987;109:8117–8119.
-
- Panek JS, Schaus JV. Tetrahedron. 1997;53:10971–10982.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

