Gene delivery in the equine cornea: a novel therapeutic strategy
- PMID: 20840107
- PMCID: PMC3711113
- DOI: 10.1111/j.1463-5224.2010.00813.x
Gene delivery in the equine cornea: a novel therapeutic strategy
Abstract
Objective: To determine if hybrid adeno-associated virus serotype 2/5 (AAV5) vector can effectively deliver foreign genes into the equine cornea without causing adverse side effects. The aims of this study were to: (i) evaluate efficacy of AAV5 to deliver therapeutic genes into equine corneal fibroblasts (ECFs) using enhanced green fluorescent protein (EGFP) marker gene, and (ii) establish the safety of AAV5 vector for equine corneal gene therapy.
Material: Primary ECF cultures were harvested from healthy donor equine corneas. Cultures were maintained at 37°C in humidified atmosphere with 5% CO(2).
Procedure: AAV5 vector expressing EGFP under control of hybrid cytomegalovirus + chicken β-actin promoter was applied topically to ECF. Expression of delivered EGFP gene in ECF was quantified using fluorescent microscopy. Using fluorescent staining, the total number of cells and transduction efficiency of tested AAV vector was determined. Phase contrast microscopy, trypan blue and TUNEL assays were used to determine toxicity and safety of AAV5 for ECFs.
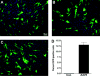
Results: Topical AAV5 application successfully transduced significant numbers of ECFs. Transduction efficiency was 13.1%. Tested AAV5 vector did not cause phenotype change or significant cell death and cell viability was maintained.
Conclusions: Tested AAV5 vector is effective and safe for gene therapy in ECFs in vitro.
Figures


References
-
- Mohan RR, Schultz GS, Hong JW, et al. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003;76:373–383. - PubMed
-
- Mohan RR, Sharma A, Netto MV, et al. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–559. - PubMed
-
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. - PubMed
-
- Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

