Desipramine, substrate for CYP2D6 activity: population pharmacokinetic model and design elements of drug-drug interaction trials
- PMID: 20840444
- PMCID: PMC2950987
- DOI: 10.1111/j.1365-2125.2010.03731.x
Desipramine, substrate for CYP2D6 activity: population pharmacokinetic model and design elements of drug-drug interaction trials
Abstract
Aims: To develop a population pharmacokinetic model to describe the pharmacokinetics of desipramine in healthy subjects, after oral administration of a 50mg dose. Additional objectives were to develop a semi-mechanistic population pharmacokinetic model for desipramine, which allowed simulation of CYP2D6-mediated inhibition, when using desipramine as a probe substrate, and to evaluate certain study design elements, such as duration of desipramine pharmacokinetic sampling, required sample size and optimal pharmacokinetic sampling schedule for intermediate, extensive and ultrarapid metabolizers of CYP2D6 substrates.
Results: The mean population estimates of the first order absorption rate constant (k(a) ), apparent clearance (CL/F) and apparent volume of distribution at steady state (V(ss) /F) were 0.15h(-1) , 111 lh(-1) and 2950 l, respectively. Further, using the proposed semi-mechanistic hepatic intrinsic clearance model with Bayesian inference, mean population desipramine hepatic intrinsic clearance was estimated to be 262 lh(-1) with between-subject variability of 84%. d-optimal PK sampling times for intermediate metabolizers were calculated to be approximately 0.25, 24, 75 and 200h. Similar sampling times were found for ultrarapid and extensive metabolizers except that the second d-optimal sample was earlier at 14 and 19h, respectively, compared with 24h for intermediate metabolizers. This difference in sampling times between the three genotypes can be attributed to the different intrinsic clearances and elimination rates.
Conclusions: A two compartment population pharmacokinetic model best described desipramine disposition. The semi-mechanistic population model developed is suitable to describe the pharmacokinetic behaviour of desipramine for the dose routinely used in drug-drug interaction (DDI) studies. Based on this meta-analysis of seven trials, a sample size of 21 subjects in cross-over design is appropriate for assessing CYP2D6 interaction with novel compounds.
© 2010 The Authors. British Journal of Clinical Pharmacology © 2010 The British Pharmacological Society.
Figures
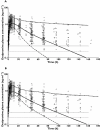
 ); 95th percentile (
); 95th percentile ( ); median (
); median ( ); data (
); data ( ); LLQ (
); LLQ ( ); LLQ:800;900 (
); LLQ:800;900 ( )
)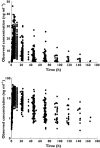


 ); UMs (
); UMs ( );
);  );
);  );
);  )
)
References
-
- Gram LF, Christiansen J. First pass metabolism of imipramine in man. Clin Pharmacol Ther. 1975;17:555–63. - PubMed
-
- Nagy A, Treiber L. Quantitative determination of imipramine and desipramine in human blood plasma by direct densitometry of thinlayer chromatograms. J Pharm Pharmacol. 1973;25:599–603. - PubMed
-
- Zeidenberg P, Perel JM, Kanzler M, Warthon RN, Malitz S. Clinical and metabolic studies with imipramine in man. Am J Psychiatry. 1971;127:1321–6. - PubMed
-
- Christiansen J, Gram LF, Kofod B, Rafaelsen OJ. Imipramine metabolism in man. Psychopharmacologia. 1967;11:255–64. - PubMed
-
- Alexanderson B. Pharmacokinetics of desmethylimipramine and nortriptyline in man after single and multiple oral doses. Eur J Clin Pharmacol. 1972;5:1–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

