ERBB receptor activation is required for profibrotic responses to transforming growth factor beta
- PMID: 20841477
- PMCID: PMC3093933
- DOI: 10.1158/0008-5472.CAN-10-0232
ERBB receptor activation is required for profibrotic responses to transforming growth factor beta
Abstract
Engagement of the transforming growth factor-β (TGF-β) receptor complex activates multiple signaling pathways that play crucial roles in both health and disease. TGF-β is a key regulator of fibrogenesis and cancer-associated desmoplasia; however, its exact mode of action in these pathologic processes has remained poorly defined. Here, we report a novel mechanism whereby signaling via members of the ERBB or epidermal growth factor family of receptors serves as a central requirement for the biological responses of fibroblasts to TGF-β. We show that TGF-β triggers upregulation of ERBB ligands and activation of cognate receptors via the canonical SMAD pathway in fibroblasts. Interestingly, activation of ERBB is commonly observed in a subset of fibroblast but not epithelial cells from different species, indicating cell type specificity. Moreover, using genetic and pharmacologic approaches, we show that ERBB activation by TGF-β is essential for the induction of fibroblast cell morphologic transformation and anchorage-independent growth. Together, these results uncover important aspects of TGF-β signaling that highlight the role of ERBB ligands/receptors as critical mediators in fibroblast responses to this pleiotropic cytokine.
© 2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

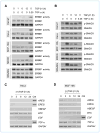

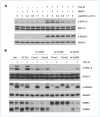
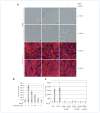
References
-
- Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat Cell Biol. 2007;9:1000–4. - PubMed
-
- Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
-
- Prud’homme GJ. Pathobiology of transforming growth factor-β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87:1077–91. - PubMed
-
- Rahimi RA, Leof EB. TGF-β signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. - PubMed
-
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

