Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway
- PMID: 20841485
- PMCID: PMC2970685
- DOI: 10.1158/0008-5472.CAN-10-1345
Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway
Abstract
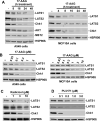
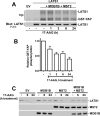
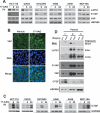
Heat shock protein 90 (HSP90), which regulates the functions of multiple oncogenic signaling pathways, has emerged as a novel anticancer therapeutic target, and multiple small-molecule HSP90 inhibitors are now in clinical trials. Although the effects of HSP90 inhibitors on oncogenic signaling pathways have been extensively studied, the effects of these agents on tumor suppressor signaling pathways are currently unknown. Here, we have examined how HSP90 inhibitors affect LATS1 and the related protein LATS2, two kinases that relay antiproliferative signals in the Hippo tumor suppressor pathway. Both LATS1 and LATS2 were depleted from cells treated with the HSP90 inhibitors 17-allylamino-17-demethoxygeldanamycin (17-AAG), radicicol, and PU-H71. Moreover, these kinases interacted with HSP90, and LATS1 isolated from 17-AAG-treated cells had reduced catalytic activity, thus showing that the kinase is a bona fide HSP90 client. Importantly, LATS1 signaling was disrupted by 17-AAG in tumor cell lines in vitro and clinical ovarian cancers in vivo as shown by reduced levels of LATS1 and decreased phosphorylation of the LATS substrate YAP, an oncoprotein transcriptional coactivator that regulates genes involved in cell and tissue growth, including the CTGF gene. Consistent with the reduced YAP phosphorylation, there were increased levels of CTGF, a secreted protein that is implicated in tumor proliferation, metastasis, and angiogenesis. Taken together, these results identify LATS1 and LATS2 as novel HSP90 clients and show that HSP90 inhibitors can disrupt the LATS tumor suppressor pathway in human cancer cells.
©2010 AACR.
Figures

References
-
- Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics - an update. Expert Opin Emerg Drugs. 2005;10:137–49. - PubMed
-
- Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–21. - PubMed
-
- Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

