Functional microRNA is transferred between glioma cells
- PMID: 20841486
- PMCID: PMC2970756
- DOI: 10.1158/0008-5472.CAN-10-0604
Functional microRNA is transferred between glioma cells
Abstract
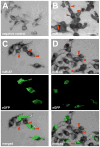
MicroRNAs (miRNA) are single-stranded 17- to 27-nucleotide RNA molecules that regulate gene expression by posttranscriptional silencing of target mRNAs. Here, we transformed rat 9L gliosarcoma cells to express cel-miR-67, a miRNA that lacks homology in rat. Coculture of these cells with cells that expressed a luciferase reporter that contained a complementary sequence to cel-miR-67 resulted in significant suppression of luciferase expression. This effect was also observed in the U87-MG human glioma cell line. Moreover, luciferase suppression was inhibited by the addition of carbenoxolone to cocultures, suggesting that gap junction communication regulates intercellular transfer of miRNA. Finally, in situ hybridization revealed the presence of cel-miR-67 in cel-miR-67-null 9L cells after coculture with cel-miR-67-expressing cells. Our data show that miRNA transcribed in glioma cells can be transferred to adjacent cells and induces targeted inhibition of protein expression in the acceptor cells. These findings reveal a novel mechanism of targeted intercellular protein regulation between brain tumor cells.
©2010 AACR.
Figures

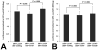
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

