Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL
- PMID: 20842052
- PMCID: PMC6322671
- DOI: 10.1097/CJI.0b013e3181f05b91
Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL
Abstract
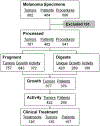
Adoptive cell transfer of tumor-infiltrating lymphocytes (TILs) can mediate objective tumor regression in 49% to 72% of patients with many long-term durable responses. To undergo treatment a patient must have (1) a resectable tumor from which (2) TIL can be generated that (3) exhibit tumor-specific reactivity. From July 2002 to July 2007, 787 tumors from 402 patients were processed for possible use in the generation of TIL, leading to the eventual treatment of 107 patients (27%). Viable TILs were generated in 376 patients (94%), and active, specific TILs were identified in 269 patients (67%). Patient demographics and tumor characteristics were analyzed for possible prognostic factors for growth and activity. Gastrointestinal-derived TIL grew less frequently, whereas lymph node and lung-derived TIL exhibited specific activity more often. TIL that grew and exhibited specific reactivity were from tumors that were larger in diameter and digests that had a higher percentage of lymphocytes. Despite these considerations, active, specific TIL could be generated from almost any site of metastasis. As more centers begin exploring the use of adoptive transfer with TIL, this compendium may provide a framework for therapeutic decision making and future investigation.
Conflict of interest statement
All authors have declared there are no financial conflicts of interest with regard to this work.
Figures

References
-
- Balch CM, Atkins MB, Sober AJ. Cutaneous melanoma In: DeVita VT Jr, Helman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:1754–1808.
-
- Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

