Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma
- PMID: 20842054
- PMCID: PMC7322622
- DOI: 10.1097/CJI.0b013e3181eec14c
Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma
Abstract
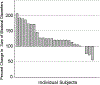
New, effective therapies are needed for pancreatic ductal adenocarcinoma. Ipilimumab can mediate an immunologic tumor regression in other histologies. This phase II trial evaluated the efficacy of Ipilimumab for advanced pancreatic cancer. Subjects were adults with locally advanced or metastatic pancreas adenocarcinoma with measurable disease, good performance status, and minimal comorbidities. Ipilimumab was administered intravenously (3.0 mg/kg every 3 wk; 4 doses/course) for a maximum of 2 courses. Response rate by response evaluation criteria in solid tumors criteria and toxicity were measured. Twenty-seven subjects were enrolled (metastatic disease: 20 and locally advanced: 7) with median age of 55 years (27 to 68 y) and good performance status (26 with Eastern Cooperative Oncology Group performance status =0 to 1). Three subjects experienced ≥ grade 3 immune-mediated adverse events (colitis:1, encephalitis:1, hypohysitis:1). There were no responders by response evaluation criteria in solid tumors criteria but a subject experienced a delayed response after initial progressive disease. In this subject, new metastases after 2 doses of Ipilimumab established progressive disease. But continued administration of the agent per protocol resulted in significant delayed regression of the primary lesion and 20 hepatic metastases. This was reflected in tumor markers normalization, and clinically significant improvement of performance status. Single agent Ipilimumab at 3.0 mg/kg/dose is ineffective for the treatment of advanced pancreas cancer. However, a significant delayed response in one subject of this trial suggests that immunotherapeutic approaches to pancreas cancer deserve further exploration.
Conflict of interest statement
All other authors have declared there are no financial conflicts of interest in regard to this work.
Figures


References
-
- Royal RE, Wolff RA, Crane CH. Pancreatic cancer In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2008:1098–1136.
-
- Bakkevold KE, Arnesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater–results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A:698–703. - PubMed
-
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. - PubMed
-
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. discussion 782–784. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

