Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1
- PMID: 20842103
- PMCID: PMC2964170
- DOI: 10.1038/emboj.2010.223
Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1
Abstract
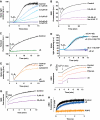
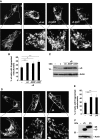
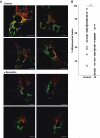
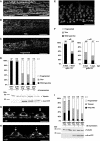
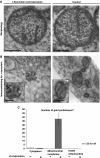
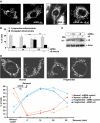
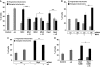
Aggregation of α-synuclein (αS) is involved in the pathogenesis of Parkinson's disease (PD) and a variety of related neurodegenerative disorders. The physiological function of αS is largely unknown. We demonstrate with in vitro vesicle fusion experiments that αS has an inhibitory function on membrane fusion. Upon increased expression in cultured cells and in Caenorhabditis elegans, αS binds to mitochondria and leads to mitochondrial fragmentation. In C. elegans age-dependent fragmentation of mitochondria is enhanced and shifted to an earlier time point upon expression of exogenous αS. In contrast, siRNA-mediated downregulation of αS results in elongated mitochondria in cell culture. αS can act independently of mitochondrial fusion and fission proteins in shifting the dynamic morphologic equilibrium of mitochondria towards reduced fusion. Upon cellular fusion, αS prevents fusion of differently labelled mitochondrial populations. Thus, αS inhibits fusion due to its unique membrane interaction. Finally, mitochondrial fragmentation induced by expression of αS is rescued by coexpression of PINK1, parkin or DJ-1 but not the PD-associated mutations PINK1 G309D and parkin Δ1-79 or by DJ-1 C106A.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

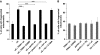
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252 - PubMed
-
- Beyer K (2007) Mechanistic aspects of Parkinson's disease. α-synuclein and the biomembrane. Cell Biochem Biophys 47: 285–299 - PubMed
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci 22: 8797–8807 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

