Short-term health-related quality of life and symptom control with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF), 5-fluorouracil (PF) for induction in unresectable locoregionally advanced head and neck cancer patients (EORTC 24971/TAX 323)
- PMID: 20842129
- PMCID: PMC2967049
- DOI: 10.1038/sj.bjc.6605860
Short-term health-related quality of life and symptom control with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF), 5-fluorouracil (PF) for induction in unresectable locoregionally advanced head and neck cancer patients (EORTC 24971/TAX 323)
Abstract
Background: The EORTC 24971/TAX 323, a phase III study of 358 patients with unresectable locoregionally advanced squamous cell carcinoma of the head and neck, showed an improved progression-free and overall survival (OS) with less toxicity when docetaxel (T) was added to cisplatin and 5-fluorouracil (PF) for induction and given before radiotherapy (RT). The impact of the addition of docetaxel on patients' health-related quality of life (HRQOL) and symptoms was investigated.
Methods: HRQOL was assessed at baseline, at end of cycle 2, and 4, 6, and 9 months after completion of RT using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) and the EORTC QLQ Head and Neck Cancer-Specific Module (EORTC QLQ-H&N35). The primary HRQOL scale was global HRQOL per protocol.
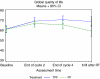
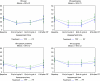
Results: Compliance to HRQOL assessments was 97% at baseline, but dropped to 54% by 6 months. Data were analysed up to 6 months. There was a trend towards improved global HRQOL during the treatment period. At 6 months after the end of RT, global HRQOL was higher in the TPF arm than in the PF arm, but the low compliance does not allow to draw definitive conclusions. Swallowing and coughing problems decreased more in the TPF arm than in the PF arm at the end of cycle 2, but to a limited extent.
Conclusion: Induction chemotherapy with TPF before RT not only improves survival and reduces toxicity compared with PF but also seems to improve global HRQOL in a more sustainable manner.
Trial registration: ClinicalTrials.gov NCT00003888.
Conflict of interest statement
JBV is consultant for Sanofi-Aventis.
Figures
References
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376 - PubMed
-
- Abdel-Wahab M, Abitbol A, Lewin A, Troner M, Hamilton K, Markoe A (2005) Quality-of-life assessment after hyperfractionated radiation therapy and 5-fluorouracil, cisplatin, and paclitaxel (Taxol) in inoperable and/or unresectable head and neck squamous cell carcinoma. Am J Clin Oncol 28: 359–366 - PubMed
-
- Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, Mercier JP, Tetu B, Harel F, Abdous B, Vigneault E, Vass S, Del VP, Roy J (2005) Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol 23: 5805–5813 - PubMed
-
- Bentzen SM, Trotti A (2007) Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol 25: 4096–4103 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical