Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST
- PMID: 20842228
- PMCID: PMC2938069
- DOI: 10.7150/jca.1.80
Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST
Abstract
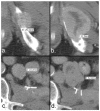
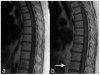
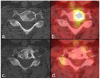
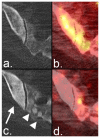
Response criteria represent the standard by which the efficacy of therapeutic agents is determined in cancer trials. The most widely used criteria are based on the anatomic measurement of solid tumors. Because bone metastases are typically located in irregularly shaped bones and are difficult to measure with rulers, they have been previously considered unmeasurable disease. New developments in cancer response criteria have increased awareness of the importance of the response of bone metastases to therapy. The recently updated Response Evaluation Criteria in Solid Tumors (RECIST 1.1) now consider bone metastases with soft tissue masses > 10 mm to be measurable disease. Response criteria specific to bone metastases have been developed at The University of Texas MD Anderson Cancer Center (MDA criteria) and can be used to assess therapeutic response in numerous types of bone metastases. Functional imaging criteria, such as the recently developed Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) allow response to be measured in the absence of anatomic change through assessment of metabolic activity. As monitoring tumor response of bone metastases becomes more important in the management of cancer, so does the demand on radiologists and nuclear medicine physicians for accurate interpretation of the behavior of these lesions. This article reviews anatomic, bone, and metabolic response criteria, providing illustrations for the interpretation of therapy-induced change in bone metastases.
Keywords: MDA; PERCIST; RECIST 1.1; bone; metastasis; response criteria.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures





References
-
- Cancer Facts & Figures 2010. American Cancer Society; http://www.cancer.org/downloads/STT/Cancer_Facts_and_Figures_2010.pdf.
-
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. - PubMed
-
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. - PubMed
-
- WHO. WHO handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization Offset Publication; 1979.
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

