Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery
- PMID: 20842541
- PMCID: PMC2974104
- DOI: 10.1208/s12249-010-9520-y
Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery
Abstract
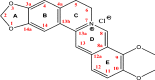
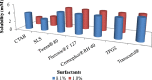
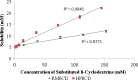
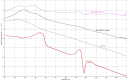
The objective of the present research was to evaluate the physicochemical characteristics of berberine chloride and to assess the complexation of drug with 2-hydroxypropyl-β-cyclodextrin (HPβCD), a first step towards solution dosage form development. The parameters such as log P value were determined experimentally and compared with predicted values. The pH-dependent aqueous solubility and stability were investigated following standard protocols at 25°C and 37°C. Drug solubility enhancement was attempted utilizing both surfactants and cyclodextrins (CDs), and the drug/CD complexation was studied employing various techniques such as differential scanning calorimetry, Fourier transform infrared, nuclear magnetic resonance, and scanning electron microscopy. The experimental log P value suggested that the compound is fairly hydrophilic. Berberine chloride was found to be very stable up to 6 months at all pH and temperature conditions tested. Aqueous solubility of the drug was temperature dependent and exhibited highest solubility of 4.05 ± 0.09 mM in phosphate buffer (pH 7.0) at 25°C, demonstrating the effect of buffer salts on drug solubility. Decreased drug solubility was observed with increasing concentrations of ionic surfactants such as sodium lauryl sulfate and cetyl trimethyl ammonium bromide. Phase solubility studies demonstrated the formation of berberine chloride-HPβCD inclusion complex with 1:1 stoichiometry, and the aqueous solubility of the drug improved almost 4.5-fold in the presence of 20% HPβCD. The complexation efficiency values indicated that the drug has at least threefold greater affinity for hydroxypropyl-β-CD compared to randomly methylated-β-CD. The characterization techniques confirmed inclusion complex formation between berberine chloride and HPβCD and demonstrated the feasibility of developing an oral solution dosage form of the drug.
Figures
Similar articles
-
Influence of hydroxypropyl-beta-cyclodextrin complexation on piroxicam release from buccoadhesive tablets.Eur J Pharm Sci. 2004 Feb;21(2-3):251-60. doi: 10.1016/j.ejps.2003.10.029. Eur J Pharm Sci. 2004. PMID: 14757497
-
Dihydroartemisinin-cyclodextrin complexation: solubility and stability.Arch Pharm Res. 2009 Jan;32(1):155-65. doi: 10.1007/s12272-009-1130-4. Epub 2009 Jan 29. Arch Pharm Res. 2009. PMID: 19183889
-
Influence of levodropropizine and hydroxypropyl-β-cyclodextrin association on the physicochemical characteristics of levodropropizine loaded in hydroxypropyl-β-cyclodextrin microcontainers: Formulation and in vitro characterization.Polim Med. 2019 Jan-Jun;49(1):35-43. doi: 10.17219/pim/111887. Polim Med. 2019. PMID: 31769938
-
Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: preparation and in-vitro evaluation.Eur J Pharm Biopharm. 2008 Nov;70(3):819-27. doi: 10.1016/j.ejpb.2008.06.024. Epub 2008 Jul 4. Eur J Pharm Biopharm. 2008. PMID: 18655829
-
Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir.J Pharm Biomed Anal. 2008 Jul 15;47(3):535-40. doi: 10.1016/j.jpba.2008.02.006. Epub 2008 Feb 15. J Pharm Biomed Anal. 2008. PMID: 18367363 Review.
Cited by
-
Preparation and Evaluation of Berberine-Excipient Complexes in Enhancing the Dissolution Rate of Berberine Incorporated into Pellet Formulations.AAPS PharmSciTech. 2024 Jul 3;25(6):154. doi: 10.1208/s12249-024-02863-1. AAPS PharmSciTech. 2024. PMID: 38961012
-
Effects of Berberine against Pancreatitis and Pancreatic Cancer.Molecules. 2022 Dec 6;27(23):8630. doi: 10.3390/molecules27238630. Molecules. 2022. PMID: 36500723 Free PMC article. Review.
-
A new pseudopolymorph of berberine chloride: crystal structure and Hirshfeld surface analysis.Acta Crystallogr E Crystallogr Commun. 2022 Apr 5;78(Pt 5):468-472. doi: 10.1107/S2056989022003309. eCollection 2022 May 1. Acta Crystallogr E Crystallogr Commun. 2022. PMID: 35547800 Free PMC article.
-
Sortase A Inhibitor Protein Nanoparticle Formulations Demonstrate Antibacterial Synergy When Combined with Antimicrobial Peptides.Molecules. 2023 Feb 24;28(5):2114. doi: 10.3390/molecules28052114. Molecules. 2023. PMID: 36903360 Free PMC article.
-
Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics.Theranostics. 2019 Mar 16;9(7):1923-1951. doi: 10.7150/thno.30787. eCollection 2019. Theranostics. 2019. PMID: 31037148 Free PMC article. Review.
References
-
- Dostál J, Man S, Seckárová P, Hulová D, Necas M, Potácek M, et al. Berberine and coptisine free bases. J Mol Struct. 2004;687(1–3):135–142. doi: 10.1016/j.molstruc.2003.09.018. - DOI
-
- Birdsall TC, Kelly GS. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;2(2):94–103.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

