Evidence for noncompetitive modulation of substrate-induced serotonin release
- PMID: 20842720
- PMCID: PMC2941209
- DOI: 10.1002/syn.20804
Evidence for noncompetitive modulation of substrate-induced serotonin release
Abstract
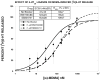
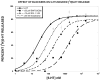
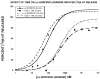
Prior work indicated that serotonin transporter (SERT) inhibitors competitively inhibit substrate-induced [(3)H]5-HT release, producing rightward shifts in the substrate-dose response curve and increasing the EC(50) value without altering the E(max). We hypothesized that this finding would not generalize across a number of SERT inhibitors and substrates, and that the functional dissociation constant (Ke) of a given SERT inhibitor would not be the same for all tested substrates. To test this hypothesis, we utilized a well-characterized [(3)H]5-HT release assay that measures the ability of a SERT substrate to release preloaded [(3)H]5-HT from rat brain synaptosomes. Dose-response curves were generated for six substrates (PAL-287 [naphthylisopropylamine], (+)-fenfluramine, (+)-norfenfluramine, mCPP [meta-chlorophenylpiperazine], (±)-MDMA, 5-HT) in the absence and presence of a fixed concentration of three SERT inhibitors (indatraline, BW723C86, EG-1-149 [4-(2-(benzhydryloxy)ethyl)-1-(4-bromobenzyl)piperidine oxalate]). Consistent with simple competitive inhibition, all SERT inhibitors increased the EC(50) value of all substrates. However, in many cases a SERT inhibitor decreased the E(max) value as well, indicating that in the presence of the SERT inhibitor the substrate became a partial releaser. Moreover, the Ke values of a given SERT inhibitor differed among the six SERT substrates, indicating that each inhibitor/substrate combination had a unique interaction with the transporter. Viewed collectively, these findings suggest that it may be possible to design SERT inhibitors that differentially regulate SERT function.
Figures



References
-
- Baumann MH, Ayestas MA, Dersch CM, Rothman RB. 1-(m-Chlorophenyl)piperazine (mCPP) Dissociates In Vivo Serotonin Release from Long-Term Serotonin Depletion in Rat Brain. Neuropsychopharmacology. 2001;24:492–501. - PubMed
-
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27(7):375–383. - PubMed
-
- Gobbi M, Funicello M, Gerstbrein K, Holy M, Moya PR, Sotomayor R, Forray MI, Gysling K, Paluzzi S, Bonanno G, Reyes-Parada M, Sitte HH, Mennini T. N,N-dimethyl-thioamphetamine and methyl-thioamphetamine, two non-neurotoxic substrates of 5-HT transporters, have scant in vitro efficacy for the induction of transporter-mediated 5-HT release and currents. J Neurochem. 2008;105(5):1770–1780. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
