Fluorine (19F) MRS and MRI in biomedicine
- PMID: 20842758
- PMCID: PMC3051284
- DOI: 10.1002/nbm.1570
Fluorine (19F) MRS and MRI in biomedicine
Abstract
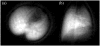
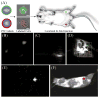
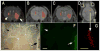
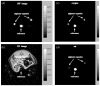
Shortly after the introduction of (1)H MRI, fluorinated molecules were tested as MR-detectable tracers or contrast agents. Many fluorinated compounds, which are nontoxic and chemically inert, are now being used in a broad range of biomedical applications, including anesthetics, chemotherapeutic agents, and molecules with high oxygen solubility for respiration and blood substitution. These compounds can be monitored by fluorine ((19)F) MRI and/or MRS, providing a noninvasive means to interrogate associated functions in biological systems. As a result of the lack of endogenous fluorine in living organisms, (19)F MRI of 'hotspots' of targeted fluorinated contrast agents has recently opened up new research avenues in molecular and cellular imaging. This includes the specific targeting and imaging of cellular surface epitopes, as well as MRI cell tracking of endogenous macrophages, injected immune cells and stem cell transplants.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures




References
-
- Holland GN, Bottomley PA, Hinshaw WS. 19F magnetic resonance imaging. J Magn Reson. 1977;28:133–136.
-
- Bulte JWM. Hot spot MRI emerges from the background. Nat Biotech. 2005;23:945–946. - PubMed
-
- Clark LC, Gollan F. Survival of mammals breathing organic liquids equilibrated with oxygen at atmospheric pressure. Science. 1966;152:1755–1756. - PubMed
-
- Clark LC, Ackerman JL, Thomas SR, Millard RW, Hoffman RE, Pratt RG, Ragle-Cole H, Kinsey RA, Janakiraman R. Perfluorinated organic liquids and emulsions as biocompatible NMR imaging agents for 19F and dissolved oxygen. Adv Exp Med Biol. 1984;180:835–845. - PubMed
-
- Thomas SR. The biomedical application of fluorine-19 NMR. In: Partain CI, Patton JA, Kulkarni MV, James AEJ, editors. Magnetic Resonance Imaging. Vol. 2. Saunders Co; London: 1988. pp. 1536–1552.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

