Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis
- PMID: 20843246
- PMCID: PMC5187954
- DOI: 10.1056/NEJMoa1002028
Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis
Abstract
Background: Myelofibrosis is a Philadelphia chromosome–negative myeloproliferative neoplasm associated with cytopenias, splenomegaly, poor quality of life, and shortened survival. About half of patients with myelofibrosis carry a gain-of-function mutation in the Janus kinase 2 gene (JAK2 V617F) that contributes to the pathophysiology of the disease. INCB018424 is a potent and selective Janus kinase 1 (JAK1) and JAK2 inhibitor.
Methods: We conducted a phase 1−2 trial of INCB018424 in patients with JAK2 V617F−positive or JAK2 V617F−negative primary myelofibrosis, post–essential thrombocythemia myelofibrosis, or post–polycythemia vera myelofibrosis.
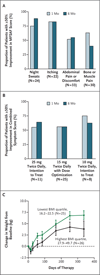
Results: A total of 153 patients received INCB018424 for a median duration of more than 14.7 months. The initial dose-escalation phase established 25 mg twice daily or 100 mg once daily as maximum tolerated doses, on the basis of reversible thrombocytopenia. A dose-dependent suppression of phosphorylated signal transducer and activator of transcription 3 (STAT3), a marker of JAK signaling, was demonstrated in patients with wild-type JAK2 and in patients with the JAK2 V617F mutation. We studied additional doses and established that a 15-mg twice-daily starting dose, followed by individualized dose titration, was the most effective and safest dosing regimen. At this dose, 17 of 33 patients (52%) had a rapid objective response (≥50% reduction of splenomegaly) lasting for 12 months or more, and this therapy was associated with grade 3 or grade 4 adverse events (mainly myelosuppression) in less than 10% of patients. Patients with debilitating symptoms, including weight loss, fatigue, night sweats, and pruritus, had rapid improvement. Clinical benefits were associated with a marked diminution of levels of circulating inflammatory cytokines that are commonly elevated in myelofibrosis.
Conclusions: INCB018424 was associated with marked and durable clinical benefits in patients with myelofibrosis for whom no approved therapies existed. (Funded by Incyte; ClinicalTrials.gov number, NCT00509899.)
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
From palliation to targeted therapy in myelofibrosis.N Engl J Med. 2010 Sep 16;363(12):1180-2. doi: 10.1056/NEJMe1005856. N Engl J Med. 2010. PMID: 20843255 No abstract available.
-
JAK inhibition in myelofibrosis.N Engl J Med. 2010 Dec 16;363(25):2464; author reply 2464-5; discussion 2465. doi: 10.1056/NEJMc1011635. N Engl J Med. 2010. PMID: 21158663 No abstract available.
References
-
- Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. - PubMed
-
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. - PubMed
-
- Cervantes F, Passamonti F, Barosi G. Life expectancy and prognostic factors in the classic BCR/ABL-negative myeloproliferative disorders. Leukemia. 2008;22:905–914. - PubMed
-
- Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. - PubMed
-
- Tefferi A. New insights into the pathogenesis and drug treatment of myelofibrosis. Curr Opin Hematol. 2006;13:87–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
