Chronic morphine treatment inhibits LPS-induced angiogenesis: implications in wound healing
- PMID: 20843508
- PMCID: PMC2950783
- DOI: 10.1016/j.cellimm.2010.08.002
Chronic morphine treatment inhibits LPS-induced angiogenesis: implications in wound healing
Abstract
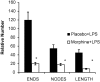
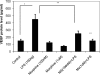
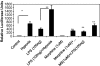
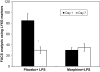
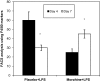
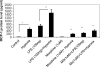
Delayed wound healing is a chronic problem in opioid drug abusers. We investigated the role chronic morphine plays on later stages of wound healing events using an angiogenesis model. Our results show that morphine treatment resulted in a significant decrease in inflammation induced angiogenesis. To delineate the mechanisms involved we investigate the role of hypoxia inducible factor 1 alpha (HIF-1 alpha), a potent inducer of angiogenic growth factor. Morphine treatment resulted in a significant decrease in the expression and nuclear translocation of HIF-1 alpha with a concurrent suppression in vascular endothelial growth factor (VEGF) synthesis. Cells of the innate immune system play a dominant role in the angiogenic process. Morphine treatment inhibited early recruitment of both neutrophils and monocytes towards an inflammatory signal with a significant decrease in the monocyte chemoattractant MCP-1. Taken together, our studies show that morphine regulates the wound repair process on multiple levels. Morphine acts both directly and indirectly in suppressing angiogenesis.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Glaucine inhibits hypoxia-induced angiogenesis and attenuates LPS-induced inflammation in human retinal pigment epithelial ARPE-19 cells.Eur J Pharmacol. 2024 Oct 15;981:176883. doi: 10.1016/j.ejphar.2024.176883. Epub 2024 Aug 10. Eur J Pharmacol. 2024. PMID: 39128809
-
Paracrine regulation of vascular endothelial growth factor--a expression during macrophage-melanoma cell interaction: role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor.J Interferon Cytokine Res. 2005 Nov;25(11):674-83. doi: 10.1089/jir.2005.25.674. J Interferon Cytokine Res. 2005. PMID: 16318581
-
Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats.Wound Repair Regen. 2019 Jul;27(4):324-334. doi: 10.1111/wrr.12708. Epub 2019 Feb 28. Wound Repair Regen. 2019. PMID: 30817065
-
The Antiangiogenesis Role of Histone Deacetylase Inhibitors: Their Potential Application to Tumor Therapy and Tissue Repair.DNA Cell Biol. 2020 Feb;39(2):167-176. doi: 10.1089/dna.2019.4877. Epub 2019 Dec 5. DNA Cell Biol. 2020. PMID: 31808715 Review.
-
Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis.Curr Drug Targets. 2008 May;9(5):422-35. doi: 10.2174/138945008784221215. Curr Drug Targets. 2008. PMID: 18473772 Review.
Cited by
-
Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections.J Neuroimmune Pharmacol. 2011 Dec;6(4):442-65. doi: 10.1007/s11481-011-9292-5. Epub 2011 Jul 26. J Neuroimmune Pharmacol. 2011. PMID: 21789507 Free PMC article. Review.
-
Morphine use in cancer surgery.Front Pharmacol. 2011 Aug 8;2:46. doi: 10.3389/fphar.2011.00046. eCollection 2011. Front Pharmacol. 2011. PMID: 21852973 Free PMC article.
-
Comparison of the effects of opioid-free anesthesia (OFA) and opioid-based anesthesia (OBA) on postoperative analgesia and intraoperative hemodynamics in patients undergoing spine surgery: A prospective randomized double-blind controlled trial.Saudi J Anaesth. 2024 Apr-Jun;18(2):173-180. doi: 10.4103/sja.sja_341_23. Epub 2024 Mar 14. Saudi J Anaesth. 2024. PMID: 38654849 Free PMC article.
-
Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia.Arthritis Res Ther. 2012 Aug 7;14(4):R181. doi: 10.1186/ar4011. Arthritis Res Ther. 2012. PMID: 22870988 Free PMC article.
-
Pain Management in Animals with Oncological Disease: Opioids as Influencers of Immune and Tumor Cellular Balance.Cancers (Basel). 2024 Aug 29;16(17):3015. doi: 10.3390/cancers16173015. Cancers (Basel). 2024. PMID: 39272873 Free PMC article. Review.
References
-
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1) alpha: Its protein stability and biological functions. Experimental & Molecular Medicine. 2004;36(1):1–12. - PubMed
-
- Bates DO, Jones RO. The role of vascular endothelial growth factor in wound healing. The International Journal of Lower Extremity Wounds. 2003;2(2):107–120. - PubMed
-
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacological Reviews. 2004;56(4):549–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

