Cell cholesterol homeostasis: mediation by active cholesterol
- PMID: 20843692
- PMCID: PMC2967630
- DOI: 10.1016/j.tcb.2010.08.007
Cell cholesterol homeostasis: mediation by active cholesterol
Abstract
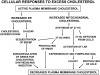
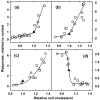
Recent evidence suggests that the major pathways mediating cell cholesterol homeostasis respond to a common signal: active membrane cholesterol. Active cholesterol is the fraction that exceeds the complexing capacity of the polar bilayer lipids. Increments in plasma membrane cholesterol exceeding this threshold have an elevated chemical activity (escape tendency) and redistribute via diverse transport proteins to both circulating plasma lipoproteins and intracellular organelles. Active cholesterol thereby prompts several feedback responses. It is the substrate for its own esterification and for the synthesis of regulatory side-chain oxysterols. It also stimulates manifold pathways that down-regulate the biosynthesis, curtail the ingestion and increase the export of cholesterol. Thus, the abundance of cell cholesterol is tightly coupled to that of its polar lipid partners through active cholesterol.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure statement. The authors have no actual or potential conflicts of interest regarding this publication.
Figures
References
-
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. - PubMed
-
- Chang TY, et al. Cholesterol Sensing, Trafficking, and Esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. - PubMed
-
- Goldstein JL, et al. Protein sensors for membrane sterols. Cell. 2006;124:35–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

