Design, construction and characterization of a set of insulated bacterial promoters
- PMID: 20843779
- PMCID: PMC3035448
- DOI: 10.1093/nar/gkq810
Design, construction and characterization of a set of insulated bacterial promoters
Abstract
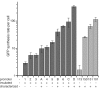
We have generated a series of variable-strength, constitutive, bacterial promoters that act predictably in different sequence contexts, span two orders of magnitude in strength and contain convenient sites for cloning and the introduction of downstream open-reading frames. Importantly, their design insulates these promoters from the stimulatory or repressive effects of many 5'- or 3'-sequence elements. We show that different promoters from our library produce constant relative levels of two different proteins in multiple genetic contexts. This set of promoters should be a useful resource for the synthetic-biology community.
Figures




References
-
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. - PubMed
-
- Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

