Against the rules: human keratin K80: two functional alternative splice variants, K80 and K80.1, with special cellular localization in a wide range of epithelia
- PMID: 20843789
- PMCID: PMC2978620
- DOI: 10.1074/jbc.M110.161745
Against the rules: human keratin K80: two functional alternative splice variants, K80 and K80.1, with special cellular localization in a wide range of epithelia
Abstract
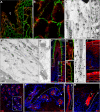
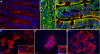
Of the 54 human keratins, five members have, at present, only been characterized at the gene level. In this study we have investigated the expression patterns of keratin K80, whose gene is located at the centromeric end of the type II keratin gene domain. K80 possesses a number of highly unusual properties. Structurally, it is distinctly closer to type II hair keratins than to type II epithelial keratins. Nonetheless, it is found in virtually all types of epithelia (stratified keratinizing/non-keratinizing, hard-keratinizing, as well as non-stratified tissues, and cell cultures thereof). This conspicuously broad expression range implies an unprecedented in vivo promiscuity of K80, which involves more than 20 different type I partners for intermediate filament (IF) formation. Throughout, K80 expression is related to advanced tissue or cell differentiation. However, instead of being part of the cytoplasmic IF network, K80 containing IFs are located at the cell margins close to the desmosomal plaques, where they are tightly interlaced with the cytoplasmic IF bundles abutting there. In contrast, in cells entering terminal differentiation, K80 adopts the "conventional" cytoplasmic distribution. In evolutionary terms, K80 is one of the oldest keratins, demonstrable down to fish. In addition, KRT80 mRNA is subject to alternative splicing. Besides K80, we describe a smaller but fully functional splice variant K80.1, which arose only during mammalian evolution. Remarkably, unlike the widely expressed K80, the expression of K80.1 is restricted to soft and hard keratinizing epithelial structures of the hair follicle and the filiform tongue papilla.
Figures



References
-
- Rogers M. A., Winter H., Langbein L., Bleiler R., Schweizer J. (2004) Differentiation 72, 527–540 - PubMed
-
- Rogers M. A., Edler L., Winter H., Langbein L., Beckmann I., Schweizer J. (2005) J. Invest. Dermatol. 124, 536–544 - PubMed
-
- Hesse M., Zimek A., Weber K., Magin T. M. (2004) Eur. J. Cell Biol. 83, 19–26 - PubMed
-
- Lu H., Zimek A., Chen J., Hesse M., Büssow H., Weber K., Magin T. M. (2006) Eur. J. Cell Biol. 85, 803–811 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

