Integrin alphaV is necessary for gastrulation movements that regulate vertebrate body asymmetry
- PMID: 20843856
- PMCID: PMC2947757
- DOI: 10.1242/dev.045310
Integrin alphaV is necessary for gastrulation movements that regulate vertebrate body asymmetry
Abstract
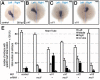
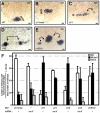
Integrin αV can form heterodimers with several β subunits to mediate cell-cell and cell-extracellular matrix interactions. During zebrafish gastrulation, αV is expressed maternally and zygotically. Here, we used a morpholino-mediated αV knockdown strategy to study αV function. Although αV morphants displayed vascular defects, they also exhibited left-right body asymmetry defects affecting multiple visceral organs. This was preceded by mislocalization of dorsal forerunner cells (DFCs) and malformation of the Kupffer's vesicle (KV) laterality organ. These defects were rescued with morpholino-resistant αV mRNA. Like αV, integrin β1b was expressed in DFCs, and β1b knockdown largely recapitulated the laterality phenotype of αV morphants. When tracked in real-time, individual DFCs of both morphants showed defects in DFC migration, preventing them from organizing into a KV of normal shape and size. Thus, we propose that αVβ1b mediates cellular interactions that are necessary for DFC clustering and movements necessary for Kupffer's vesicle formation, uncovering an early contribution of integrins to the regulation of vertebrate laterality.
Figures






References
-
- Ablooglu A. J., Kang J., Handin R. I., Traver D., Shattil S. J. (2007). The zebrafish vitronectin receptor: characterization of integrin alphaV and beta3 expression patterns in early vertebrate development. Dev. Dyn. 236, 2268-2276 - PubMed
-
- Alexander J., Stainier D. Y. (1999). A molecular pathway leading to endoderm formation in zebrafish. Curr. Biol. 9, 1147-1157 - PubMed
-
- Amack J. D., Yost H. J. (2004). The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr. Biol. 14, 685-690 - PubMed
-
- Amack J. D., Wang X., Yost H. J. (2007). Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish. Dev. Biol. 310, 196-210 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

