A prominent requirement for single-minded and the ventral midline in patterning the dorsoventral axis of the crustacean Parhyale hawaiensis
- PMID: 20843860
- PMCID: PMC2947759
- DOI: 10.1242/dev.055160
A prominent requirement for single-minded and the ventral midline in patterning the dorsoventral axis of the crustacean Parhyale hawaiensis
Abstract
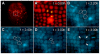
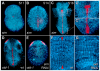
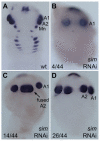
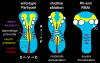
In bilaterians, establishing the correct spatial positioning of structures along the dorsoventral (DV) axis is essential for proper embryonic development. Insects such as Drosophila rely on the Dorsal activity gradient and Bone morphogenetic protein (BMP) signaling to establish cell fates along the DV axis, leading to the distinction between tissues such as mesoderm, neurogenic ectoderm and dorsal ectoderm in the developing embryo. Subsequently, the ventral midline plays a more restricted role in DV patterning by establishing differential cell fates in adjacent regions of the neurogenic ectoderm. In this study, we examine the function of the ventral midline and the midline-associated gene single-minded (Ph-sim) in the amphipod crustacean Parhyale hawaiensis. Remarkably, we found that Ph-sim and the ventral midline play a central role in establishing proper fates along the entire DV axis in this animal; laser ablation of midline cells causes a failure to form neurogenic ectoderm and Ph-sim RNAi results in severely dorsalized embryos lacking both neurogenic ectoderm and the appendage-bearing lateral ectoderm. Furthermore, we hypothesize that this role of midline cells was present in the last common ancestor of crustaceans and insects. We predict that the transition to a Dorsal-dependent DV patterning system in the phylogenetically derived insect lineage leading to Drosophila has led to a more restricted role of the ventral midline in patterning the DV axis of these insects.
Figures


References
-
- Akiyama-Oda Y., Oda H. (2006). Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development 133, 2347-2357 - PubMed
-
- Browne W. E., Price A. L., Gerberding M., Patel N. H. (2005). Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 42, 124-149 - PubMed
-
- Browne W. E., Schmid B. G., Wimmer E. A., Martindale M. Q. (2006). Expression of otd orthologs in the amphipod crustacean, Parhyale hawaiensis. Dev. Genes Evol. 216, 581-595 - PubMed
-
- Brusca R. C., Brusca G. J. (2003). Invertebrates. Sunderland, MA: Sinauer Associates;
-
- Chang J., Kim I. O., Ahn J. S., Kwon J. S., Jeon S. H., Kim S. H. (2000). The CNS midline cells coordinate proper cell cycle progression and identity determination of the Drosophila ventral neuroectoderm. Dev. Biol. 227, 307-323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

