Low allergenic potential with fondaparinux: results of a prospective investigation
- PMID: 20843983
- PMCID: PMC2947963
- DOI: 10.4065/mcp.2010.0346
Low allergenic potential with fondaparinux: results of a prospective investigation
Abstract
Objective: To determine the incidence and causes of skin reactions to the synthetic pentasaccharide fondaparinux.
Patients and methods: Patients who received prophylactic/therapeutic subcutaneous fondaparinux treatment for more than 7 days were prospectively examined for cutaneous adverse effects between September 1, 2008, and April 30, 2009. When indicated, other procedures, such as skin biopsy, allergy testing, and clinical/laboratory assessment for thrombosis and heparin-induced thrombocytopenia, were performed.
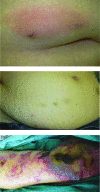
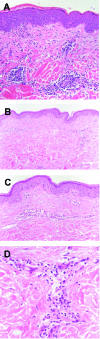
Results: Overall, 231 patients were enrolled. No patient developed typical delayed type IV hypersensitivity (DTH) erythematous skin lesions. However, one female patient experienced abdominal pruritus at sites of injection. Histology revealed a mild lymphohistiocytic infiltrate, confirming a DTH reaction. Heparin-induced thrombocytopenia, as another possible underlying pathomechanism for cutaneous lesions, was ruled out clinically and serologically. Hence, the overall incidence of fondaparinux-induced allergic skin lesions was 0.4% (95% confidence interval, 0.01%-2.4%). No cross-allergies were observed in patients with DTH reaction to heparins.
Conclusion: Fondaparinux has a low allergenic potential. The incidence of allergic cutaneous DTH reactions is almost 20 times lower compared to that with commonly used heparins. These results, together with the known low prevalence of secondary thrombotic events or heparin-induced thrombocytopenia during fondaparinux therapy, suggest that in selected patients fondaparinux might substantially improve patient care, therapeutic safety, and cost-effectiveness of anticoagulant therapy.
Trial registration: clinicaltrials.gov identifier: NCT00510432.
Figures
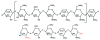
Similar articles
-
Delayed-Type Heparin Allergy: Intravenous Tolerance Despite Inflammatory Skin Reaction After Subcutaneous Injection.J Allergy Clin Immunol Pract. 2022 Nov;10(11):2977-2983.e1. doi: 10.1016/j.jaip.2022.06.030. Epub 2022 Jul 2. J Allergy Clin Immunol Pract. 2022. PMID: 35788063
-
Molecular weight determines the frequency of delayed type hypersensitivity reactions to heparin and synthetic oligosaccharides.Thromb Haemost. 2005 Dec;94(6):1265-9. doi: 10.1160/TH05-05-0318. Thromb Haemost. 2005. PMID: 16411404 Clinical Trial.
-
High incidence of heparin-induced allergic delayed-type hypersensitivity reactions in pregnancy.J Allergy Clin Immunol. 2013 Jul;132(1):131-9. doi: 10.1016/j.jaci.2013.02.047. Epub 2013 May 30. J Allergy Clin Immunol. 2013. PMID: 23726261
-
Non-immediate heparin and heparinoid cutaneous allergic reactions: a role for fondaparinux.Intern Med J. 2018 Jan;48(1):73-77. doi: 10.1111/imj.13659. Intern Med J. 2018. PMID: 29314514 Review.
-
Hypersensitivity reactions to heparins.Curr Opin Allergy Clin Immunol. 2016 Aug;16(4):315-22. doi: 10.1097/ACI.0000000000000281. Curr Opin Allergy Clin Immunol. 2016. PMID: 27285488 Review.
Cited by
-
Sustained drug-related reaction with eosinophilia and systemic symptoms (DRESS) triggered by low molecular weight heparins in COVID-19: management and precision diagnosis.Postepy Dermatol Alergol. 2022 Aug;39(4):816-818. doi: 10.5114/ada.2021.109586. Epub 2021 Oct 1. Postepy Dermatol Alergol. 2022. PMID: 36090719 Free PMC article. No abstract available.
-
Fondaparinux Pre-, Peri-, and/or Postpartum for the Prophylaxis/Treatment of Venous Thromboembolism (FondaPPP).Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211014575. doi: 10.1177/10760296211014575. Clin Appl Thromb Hemost. 2021. PMID: 33942675 Free PMC article.
References
-
- Petitou M, Lormeau JC, Choay J. Chemical synthesis of glycosaminoglycans: new approaches to antithrombotic drugs. Nature. 1991;350(6319, suppl):30-33 - PubMed
-
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6, suppl):454S-545S - PubMed
-
- Harrington RA, Becker RC, Cannon CP, et al. Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6, suppl):670S-707S - PubMed
-
- Ludwig RJ, Schindewolf M, Utikal J, Lindhoff-Last E, Boehncke WH. Management of cutaneous type IV hypersensitivity reactions induced by heparin. Thromb Haemost. 2006;96(5):611-617 - PubMed
-
- Trautmann A, Seitz CS. Heparin allergy: delayed-type non-IgE-mediated allergic hypersensitivity to subcutaneous heparin injection. Immunol Allergy Clin North Am. 2009;29(3):469-480 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

