Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues
- PMID: 20843997
- PMCID: PMC2954716
- DOI: 10.1210/en.2010-0666
Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues
Abstract
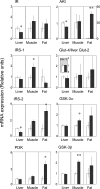
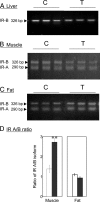
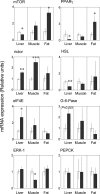
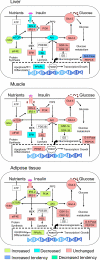
Polycystic ovarian syndrome (PCOS) is the leading cause of infertility in reproductive-aged women with the majority manifesting insulin resistance. To delineate the causes of insulin resistance in women with PCOS, we determined changes in the mRNA expression of insulin receptor (IR) isoforms and members of its signaling pathway in tissues of adult control (n = 7) and prenatal testosterone (T)-treated (n = 6) sheep (100 mg/kg twice a week from d 30-90 of gestation), the reproductive/metabolic characteristics of which are similar to women with PCOS. Findings revealed that prenatal T excess reduced (P < 0.05) expression of IR-B isoform (only isoform detected), insulin receptor substrate-2 (IRS-2), protein kinase B (AKt), peroxisome proliferator-activated receptor-γ (PPARγ), hormone-sensitive lipase (HSL), and mammalian target of rapamycin (mTOR) but increased expression of rapamycin-insensitive companion of mTOR (rictor), and eukaryotic initiation factor 4E (eIF4E) in the liver. Prenatal T excess increased (P < 0.05) the IR-A to IR-B isoform ratio and expression of IRS-1, glycogen synthase kinase-3α and -β (GSK-3α and -β), and rictor while reducing ERK1 in muscle. In the adipose tissue, prenatal T excess increased the expression of IRS-2, phosphatidylinositol 3-kinase (PI3K), PPARγ, and mTOR mRNAs. These findings provide evidence that prenatal T excess modulates in a tissue-specific manner the expression levels of several genes involved in mediating insulin action. These changes are consistent with the hypothesis that prenatal T excess disrupts the insulin sensitivity of peripheral tissues, with liver and muscle being insulin resistant and adipose tissue insulin sensitive.
Figures
References
-
- Franks S 1995 Polycystic ovary syndrome. N Engl J Med 333:853–861 - PubMed
-
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 - PubMed
-
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 - PubMed
-
- Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A 2005 Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab 288:E1047–E1054 - PubMed
-
- Book CB, Dunaif A 1999 Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 84:3110–3116 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

