Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency
- PMID: 20844025
- PMCID: PMC2977993
- DOI: 10.1530/EJE-10-0764
Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency
Abstract
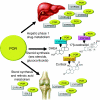
Objective: Patients with congenital adrenal hyperplasia due to P450 oxidoreductase (POR) deficiency (ORD) present with disordered sex development and glucocorticoid deficiency. This is due to disruption of electron transfer from mutant POR to microsomal cytochrome P450 (CYP) enzymes that play a key role in glucocorticoid and sex steroid synthesis. POR also transfers electrons to all major drug-metabolizing CYP enzymes, including CYP3A4 that inactivates glucocorticoid and oestrogens. However, whether ORD results in impairment of in vivo drug metabolism has never been studied.
Design: We studied an adult patient with ORD due to homozygous POR A287P, the most frequent POR mutation in Caucasians, and her clinically unaffected, heterozygous mother. The patient had received standard dose oestrogen replacement from 17 until 37 years of age when it was stopped after she developed breast cancer.
Methods: Both subjects underwent in vivo cocktail phenotyping comprising the oral administration of caffeine, tolbutamide, omeprazole, dextromethorphan hydrobromide and midazolam to assess the five major drug-metabolizing CYP enzymes. We also performed genotyping for variant CYP alleles known to affect drug metabolism.
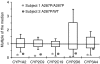
Results: Though CYP enzyme genotyping predicted normal or high enzymatic activities in both subjects, in vivo assessment showed subnormal activities of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in the patient and of CYP1A2 and CYP2C9 in her mother.
Conclusions: Our results provide in vivo evidence for an important role of POR in regulating drug metabolism and detoxification. In patients with ORD, in vivo assessment of drug-metabolizing activities with subsequent tailoring of drug therapy and steroid replacement should be considered.
Figures
Similar articles
-
A rare cause of congenital adrenal hyperplasia: Antley-Bixler syndrome due to POR deficiency.Neth J Med. 2011 Jun;69(6):281-3. Neth J Med. 2011. PMID: 21868813
-
Reduction in hepatic drug metabolizing CYP3A4 activities caused by P450 oxidoreductase mutations identified in patients with disordered steroid metabolism.Biochem Biophys Res Commun. 2010 Oct 8;401(1):149-53. doi: 10.1016/j.bbrc.2010.09.035. Epub 2010 Sep 16. Biochem Biophys Res Commun. 2010. PMID: 20849814
-
P450 oxidoreductase deficiency: a disorder of steroidogenesis with multiple clinical manifestations.Sci Signal. 2012 Oct 23;5(247):pt11. doi: 10.1126/scisignal.2003318. Sci Signal. 2012. PMID: 23092891 Review.
-
Pubertal presentation in seven patients with congenital adrenal hyperplasia due to P450 oxidoreductase deficiency.J Clin Endocrinol Metab. 2011 Mar;96(3):E453-62. doi: 10.1210/jc.2010-1607. Epub 2010 Dec 29. J Clin Endocrinol Metab. 2011. PMID: 21190981 Free PMC article.
-
Cytochrome P450 oxidoreductase deficiency: rare congenital disorder leading to skeletal malformations and steroidogenic defects.Pediatr Int. 2014 Dec;56(6):805-808. doi: 10.1111/ped.12518. Pediatr Int. 2014. PMID: 25294558 Review.
Cited by
-
Electron transfer by human wild-type and A287P mutant P450 oxidoreductase assessed by transient kinetics: functional basis of P450 oxidoreductase deficiency.Biochem J. 2015 May 15;468(1):25-31. doi: 10.1042/BJ20141410. Biochem J. 2015. PMID: 25728647 Free PMC article.
-
A limited sampling strategy based on maximum a posteriori Bayesian estimation for a five-probe phenotyping cocktail.Eur J Clin Pharmacol. 2016 Jan;72(1):39-51. doi: 10.1007/s00228-015-1953-5. Eur J Clin Pharmacol. 2016. PMID: 26490357 Clinical Trial.
-
Diagnostic challenges and management advances in cytochrome P450 oxidoreductase deficiency, a rare form of congenital adrenal hyperplasia, with 46, XX karyotype.Front Endocrinol (Lausanne). 2023 Aug 11;14:1226387. doi: 10.3389/fendo.2023.1226387. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635957 Free PMC article. Review.
-
Steroid Metabolome Analysis in Disorders of Adrenal Steroid Biosynthesis and Metabolism.Endocr Rev. 2019 Dec 1;40(6):1605-1625. doi: 10.1210/er.2018-00262. Endocr Rev. 2019. PMID: 31294783 Free PMC article.
-
Congenital adrenal hyperplasia, disorders of sex development, and infertility in patients with POR gene pathogenic variants: a systematic review of the literature.J Endocrinol Invest. 2023 Jan;46(1):1-14. doi: 10.1007/s40618-022-01849-9. Epub 2022 Jul 17. J Endocrinol Invest. 2023. PMID: 35842891 Free PMC article.
References
-
- Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363:2128–2135. doi: 10.1016/S0140-6736(04)16503-3. - DOI - PubMed
-
- Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, Okuyama T, Nakai H, Soneda S, Tachibana K, Matsuo N, Sato S, Homma K, Nishimura G, Hasegawa T, Ogata T. Cytochrome P450 oxidoreductase gene mutations and Antley–Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. Journal of Clinical Endocrinology and Metabolism. 2005;90:414–426. doi: 10.1210/jc.2004-0810. - DOI - PubMed
-
- Schmidt K, Hughes C, Chudek JA, Goodyear SR, Aspden RM, Talbot R, Gundersen TE, Blomhoff R, Henderson C, Wolf CR, Tickle C. Cholesterol metabolism: the main pathway acting downstream of cytochrome P450 oxidoreductase in skeletal development of the limb. Molecular and Cellular Biology. 2009;29:2716–2729. doi: 10.1128/MCB.01638-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

