Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication
- PMID: 20844027
- PMCID: PMC2976418
- DOI: 10.1128/JVI.00260-10
Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication
Abstract
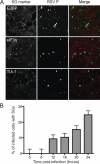
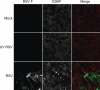
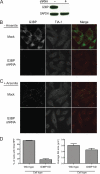
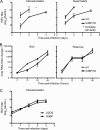
Mammalian cell cytoplasmic RNA stress granules are induced during various conditions of stress and are strongly associated with regulation of host mRNA translation. Several viruses induce stress granules during the course of infection, but the exact function of these structures during virus replication is not well understood. In this study, we showed that respiratory syncytial virus (RSV) induced host stress granules in epithelial cells during the course of infection. We also showed that stress granules are distinct from cytoplasmic viral inclusion bodies and that the RNA binding protein HuR, normally found in stress granules, also localized to viral inclusion bodies during infection. Interestingly, we demonstrated that infected cells containing stress granules also contained more RSV protein than infected cells that did not form inclusion bodies. To address the role of stress granule formation in RSV infection, we generated a stable epithelial cell line with reduced expression of the Ras-GAP SH3 domain-binding protein (G3BP) that displayed an inhibited stress granule response. Surprisingly, RSV replication was impaired in these cells compared to its replication in cells with intact G3BP expression. In contrast, knockdown of HuR by RNA interference did not affect stress granule formation or RSV replication. Finally, using RNA probes specific for RSV genomic RNA, we found that viral RNA predominantly localized to viral inclusion bodies but a small percentage also interacted with stress granules during infection. These results suggest that RSV induces a host stress granule response and preferentially replicates in host cells that have committed to a stress response.
Figures


References
-
- Brown, G., H. W. Rixon, J. Steel, T. P. McDonald, A. R. Pitt, S. Graham, and R. J. Sugrue. 2005. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology 338:69-80. - PubMed
-
- Carromeu, C., F. M. Simabuco, R. E. Tamura, L. E. Farinha Arcieri, and A. M. Ventura. 2007. Intracellular localization of human respiratory syncytial virus L protein. Arch. Virol. 152:2259-2263. - PubMed
-
- Cristea, I. M., J. W. Carroll, M. P. Rout, C. M. Rice, B. T. Chait, and M. R. MacDonald. 2006. Tracking and elucidating alphavirus-host protein interactions. J. Biol. Chem. 281:30269-30278. - PubMed
-
- Dang, Y., N. Kedersha, W. K. Low, D. Romo, M. Gorospe, R. Kaufman, P. Anderson, and J. O. Liu. 2006. Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A. J. Biol. Chem. 281:32870-32878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

