Extracellular 2'-5' oligoadenylate synthetase stimulates RNase L-independent antiviral activity: a novel mechanism of virus-induced innate immunity
- PMID: 20844035
- PMCID: PMC2977878
- DOI: 10.1128/JVI.01003-10
Extracellular 2'-5' oligoadenylate synthetase stimulates RNase L-independent antiviral activity: a novel mechanism of virus-induced innate immunity
Abstract
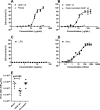
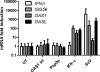
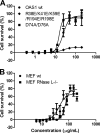
The 2'-5' oligoadenylate synthetase (OAS) proteins are traditionally considered intracellular antiviral proteins. However, several studies demonstrate a correlation between the concentration of freely circulating OAS protein in sera from hepatitis C patients and their clinical prognosis. Here we demonstrate that extracellular OAS1 enters into cells and possesses a strong antiviral activity, both in vitro and in vivo, which is independent of RNase L. The OAS protein directly inhibits viral proliferation and does not require the activation of known antiviral signaling pathways. We propose that OAS produced by cells infected with viruses is released to the extracellular space, where it acts as a paracrine antiviral agent. Thus, the OAS protein represents the first direct antiviral compound released by virus-infected cells.
Figures
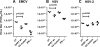

References
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

