Virulence-associated substitution D222G in the hemagglutinin of 2009 pandemic influenza A(H1N1) virus affects receptor binding
- PMID: 20844044
- PMCID: PMC2977876
- DOI: 10.1128/JVI.01136-10
Virulence-associated substitution D222G in the hemagglutinin of 2009 pandemic influenza A(H1N1) virus affects receptor binding
Abstract
The clinical impact of the 2009 pandemic influenza A(H1N1) virus (pdmH1N1) has been relatively low. However, amino acid substitution D222G in the hemagglutinin of pdmH1N1 has been associated with cases of severe disease and fatalities. D222G was introduced in a prototype pdmH1N1 by reverse genetics, and the effect on virus receptor binding, replication, antigenic properties, and pathogenesis and transmission in animal models was investigated. pdmH1N1 with D222G caused ocular disease in mice without further indications of enhanced virulence in mice and ferrets. pdmH1N1 with D222G retained transmissibility via aerosols or respiratory droplets in ferrets and guinea pigs. The virus displayed changes in attachment to human respiratory tissues in vitro, in particular increased binding to macrophages and type II pneumocytes in the alveoli and to tracheal and bronchial submucosal glands. Virus attachment studies further indicated that pdmH1N1 with D222G acquired dual receptor specificity for complex α2,3- and α2,6-linked sialic acids. Molecular dynamics modeling of the hemagglutinin structure provided an explanation for the retention of α2,6 binding. Altered receptor specificity of the virus with D222G thus affected interaction with cells of the human lower respiratory tract, possibly explaining the observed association with enhanced disease in humans.
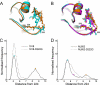
Figures

References
-
- Bayly, C. I., P. Cieplak, W. D. Cornell, and P. A. Kollman. 1993. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model J. Phys. Chem. 97:10269-10280.
-
- Calleja, M., L. Blanshard, R. Bruin, C. Chapman, A. Thandavan, R. Tyer, P. Wilson, V. Alexandrov, R. J. Allen, J. Brodholt, M. T. Dove, W. Emmerich, and K. Kleese van Dam. 2004. Grid tool integration within the eMinerals project. Proceedings of the UK e-Science All Hands Meeting 2004. ISBN 1904425216:812-817.
-
- Cao, B., X. W. Li, Y. Mao, J. Wang, H. Z. Lu, Y. S. Chen, Z. A. Liang, L. Liang, S. J. Zhang, B. Zhang, L. Gu, L. H. Lu, D. Y. Wang, C. Wang, and the National Influenza A Pandemic (H1H1) 2009 Clinical Investigation Group of China. 2009. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N. Engl. J. Med. 361:2507-2517. - PubMed
-
- Case, D. A., T. A. Darden, I. Cheatham, C. L. Simmerling, J. Wang, R. E. Duke, R. Luo, M. Crowley, R. C. Walker, W. Zhang, K. M. Merz, B. Wang, S. Hayik, A. Roitberg, G. Seabra, I. Kolossváry, K. F. Wong, F. Paesani, J. Vanicek, X. Wu, S. R. Brozell, T. Steinbrecher, H. Gohlke, L. Yang, C. Tan, J. Mongan, V. Hornak, G. Cui, D. H. Mathews, M. G. Seetin, C. Sagui, V. Babin, and P. A. Kollman. 2008. AMBER 10. University of California, San Francisco.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

