Nuclear export of human papillomavirus type 31 E1 is regulated by Cdk2 phosphorylation and required for viral genome maintenance
- PMID: 20844047
- PMCID: PMC2977856
- DOI: 10.1128/JVI.01445-10
Nuclear export of human papillomavirus type 31 E1 is regulated by Cdk2 phosphorylation and required for viral genome maintenance
Abstract
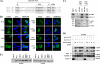
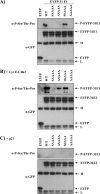
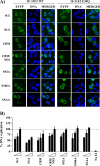
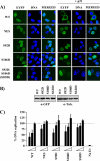
The initiator protein E1 from human papillomavirus (HPV) is a helicase essential for replication of the viral genome. E1 contains three functional domains: a C-terminal enzymatic domain that has ATPase/helicase activity, a central DNA-binding domain that recognizes specific sequences in the origin of replication, and a N-terminal region necessary for viral DNA replication in vivo but dispensable in vitro. This N-terminal portion of E1 contains a conserved nuclear export signal (NES) whose function in the viral life cycle remains unclear. In this study, we provide evidence that nuclear export of HPV31 E1 is inhibited by cyclin E/A-Cdk2 phosphorylation of two serines residues, S92 and S106, located near and within the E1 NES, respectively. Using E1 mutant proteins that are confined to the nucleus, we determined that nuclear export of E1 is not essential for transient viral DNA replication but is important for the long-term maintenance of the HPV episome in undifferentiated keratinocytes. The findings that E1 nuclear export is not required for viral DNA replication but needed for genome maintenance over multiple cell divisions raised the possibility that continuous nuclear accumulation of E1 is detrimental to cellular growth. In support of this possibility, we observed that nuclear accumulation of E1 dramatically reduces cellular proliferation by delaying cell cycle progression in S phase. On the basis of these results, we propose that nuclear export of E1 is required, at least in part, to limit accumulation of this viral helicase in the nucleus in order to prevent its detrimental effect on cellular proliferation.
Figures




References
-
- Amin, A. A., S. Titolo, A. Pelletier, D. Fink, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the HPV11 E1 protein required for DNA replication in vitro. Virology 272:137-150. - PubMed
-
- Androphy, E. J., D. R. Lowy, and J. T. Schiller. 1987. Bovine papillomavirus E2 transactivating gene product binds to specific sites in papillomavirus DNA. Nature 325:70-73. - PubMed
-
- Chow, L. T., and R. Broker. 1997. Small DNA tumor viruses, p. 267-301. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

