Autocrine transforming growth factor-{beta}1 activation mediated by integrin {alpha}V{beta}3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells
- PMID: 20844080
- PMCID: PMC2965683
- DOI: 10.1091/mbc.E10-06-0523
Autocrine transforming growth factor-{beta}1 activation mediated by integrin {alpha}V{beta}3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells
Abstract
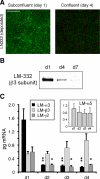
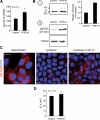
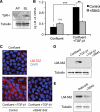
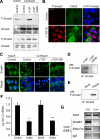
Laminin (LM)-332 is an extracellular matrix protein that plays a structural role in normal tissues and is also important in facilitating recovery of epithelia from injury. We have shown that expression of LM-332 is up-regulated during renal epithelial regeneration after ischemic injury, but the molecular signals that control expression are unknown. Here, we demonstrate that in Madin-Darby canine kidney (MDCK) epithelial cells LM-332 expression occurs only in subconfluent cultures and is turned-off after a polarized epithelium has formed. Addition of active transforming growth factor (TGF)-β1 to confluent MDCK monolayers is sufficient to induce transcription of the LM α3 gene and LM-332 protein expression via the TGF-β type I receptor (TβR-I) and the Smad2-Smad4 complex. Significantly, we show that expression of LM-332 in MDCK cells is an autocrine response to endogenous TGF-β1 secretion and activation mediated by integrin αVβ3 because neutralizing antibodies block LM-332 production in subconfluent cells. In confluent cells, latent TGF-β1 is secreted apically, whereas TβR-I and integrin αVβ3 are localized basolaterally. Disruption of the epithelial barrier by mechanical injury activates TGF-β1, leading to LM-332 expression. Together, our data suggest a novel mechanism for triggering the production of LM-332 after epithelial injury.
Figures
Similar articles
-
Activation of the ALK-5 Pathway is not per se Sufficient for the Antiproliferative Effect of TGF-β1 on Renal Tubule Epithelial Cells.Cell Physiol Biochem. 2015;37(4):1231-9. doi: 10.1159/000430246. Epub 2015 Oct 5. Cell Physiol Biochem. 2015. PMID: 26431052
-
Regulated synthesis and functions of laminin 5 in polarized madin-darby canine kidney epithelial cells.Mol Biol Cell. 2006 Aug;17(8):3664-77. doi: 10.1091/mbc.e05-11-1070. Epub 2006 Jun 14. Mol Biol Cell. 2006. PMID: 16775009 Free PMC article.
-
Loss of beta1-integrin enhances TGF-beta1-induced collagen expression in epithelial cells via increased alphavbeta3-integrin and Rac1 activity.J Biol Chem. 2010 Oct 1;285(40):30741-51. doi: 10.1074/jbc.M110.105700. Epub 2010 Jul 22. J Biol Chem. 2010. PMID: 20650890 Free PMC article.
-
Activation of extracellular signal-regulated kinase by TGF-beta1 via TbetaRII and Smad7 dependent mechanisms in human bronchial epithelial BEP2D cells.Cell Biol Toxicol. 2007 Mar;23(2):113-28. doi: 10.1007/s10565-006-0097-x. Epub 2006 Nov 9. Cell Biol Toxicol. 2007. PMID: 17096210
-
Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro.BMC Cell Biol. 2010 May 5;11:31. doi: 10.1186/1471-2121-11-31. BMC Cell Biol. 2010. PMID: 20441599 Free PMC article.
Cited by
-
Vectorial secretion of CTGF as a cell-type specific response to LPA and TGF-β in human tubular epithelial cells.Cell Commun Signal. 2012 Sep 2;10(1):25. doi: 10.1186/1478-811X-10-25. Cell Commun Signal. 2012. PMID: 22938209 Free PMC article.
-
Transforming growth factor β1 enhances adhesion of endometrial cells to mesothelium by regulating integrin expression.BMB Rep. 2017 Aug;50(8):429-434. doi: 10.5483/bmbrep.2017.50.8.097. BMB Rep. 2017. PMID: 28760197 Free PMC article.
-
Bidirectional signalling between EphA2 and ephrinA1 increases tubular cell attachment, laminin secretion and modulates erythropoietin expression after renal hypoxic injury.Pflugers Arch. 2016 Aug;468(8):1433-48. doi: 10.1007/s00424-016-1838-1. Epub 2016 May 26. Pflugers Arch. 2016. PMID: 27228995
-
Laminin 511 partners with laminin 332 to mediate directional migration of Madin-Darby canine kidney epithelial cells.Mol Biol Cell. 2012 Jan;23(1):121-36. doi: 10.1091/mbc.E11-08-0718. Epub 2011 Oct 26. Mol Biol Cell. 2012. PMID: 22031290 Free PMC article.
-
αVβ6 integrin promotes corneal wound healing.Invest Ophthalmol Vis Sci. 2011 Oct 31;52(11):8505-13. doi: 10.1167/iovs.11-8194. Invest Ophthalmol Vis Sci. 2011. PMID: 21960555 Free PMC article.
References
-
- Aberdam D., Virolle T., Simon-Assmann P. Transcriptional regulation of laminin gene expression. Microsc. Res. Tech. 2000;51:228–237. - PubMed
-
- Aumailley M., et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. - PubMed
-
- Aumailley M., El Khal A., Knoss N., Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003;22:49–54. - PubMed
-
- Basile D. P., Martin D. R., Hammerman M. R. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-β in repair. Am. J. Physiol. 1998;275:F894–F903. - PubMed
-
- Brown K. A., Pietenpol J. A., Moses H. L. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-β signaling. J. Cell. Biochem. 2007;101:9–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

