Adenomatous polyposis coli and hypoxia-inducible factor-1{alpha} have an antagonistic connection
- PMID: 20844082
- PMCID: PMC2965681
- DOI: 10.1091/mbc.E10-04-0312
Adenomatous polyposis coli and hypoxia-inducible factor-1{alpha} have an antagonistic connection
Abstract
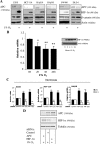
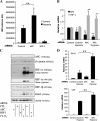
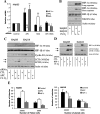
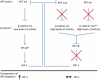
The tumor suppressor adenomatous polyposis coli (APC) is mutated in the majority of colorectal cancers and is best known for its role as a scaffold in a Wnt-regulated protein complex that determines the availability of β-catenin. Another common feature of solid tumors is the presence of hypoxia as indicated by the up-regulation of hypoxia-inducible factors (HIFs) such as HIF-1α. Here, we demonstrate a novel link between APC and hypoxia and show that APC and HIF-1α antagonize each other. Hypoxia results in reduced levels of APC mRNA and protein via a HIF-1α-dependent mechanism. HIF-1α represses the APC gene via a functional hypoxia-responsive element on the APC promoter. In contrast, APC-mediated repression of HIF-1α requires wild-type APC, low levels of β-catenin, and nuclear factor-κB activity. These results reveal down-regulation of APC as a new mechanism that contributes to the survival advantage induced by hypoxia and also show that loss of APC mutations produces a survival advantage by mimicking hypoxic conditions.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

