Functional role of the interaction between polysialic acid and extracellular histone H1
- PMID: 20844135
- PMCID: PMC6633434
- DOI: 10.1523/JNEUROSCI.6407-09.2010
Functional role of the interaction between polysialic acid and extracellular histone H1
Abstract
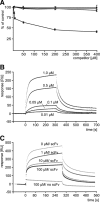
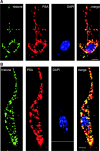
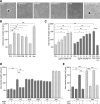
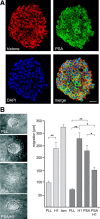
Polysialic acid (PSA) is a large and highly negatively charged glycan that plays crucial roles in nervous system development and function in the adult. It has been suggested to facilitate cell migration, neurite outgrowth, and synaptic plasticity because its hydration volume could enhance flexibility of cell interactions. Evidence for receptors of PSA has so far been elusive. We now identified histone H1 as binding partner of PSA via a single-chain variable fragment antibody using an anti-idiotypic approach. Histone H1 directly binds to PSA as shown by ELISA. Surface biotinylation of cultured cerebellar neurons indicated an extracellular localization of histone H1. Immunostaining of live cerebellar neurons and Schwann cells confirmed that an extracellular pool of histone H1 colocalizes with PSA at the cell surface. Histone H1 was also detected in detergent-insoluble synaptosomal membrane subfractions and postsynaptic densities. When applied in vitro, histone H1 stimulated neuritogenesis, process formation and proliferation of Schwann cells, and migration of neural precursor cells via a PSA-dependent mechanism, further indicating that histone H1 is active extracellularly. These in vitro observations suggested an important functional role for the interaction between histone H1 and PSA not only for nervous system development but also for regeneration in the adult. Indeed, histone H1 improved functional recovery, axon regrowth, and precision of reinnervation of the motor branch in adult mice with femoral nerve injury. Our findings encourage investigations on the therapeutic potential of histone H1 in humans.
Figures





Similar articles
-
Histone H1 improves regeneration after mouse spinal cord injury and changes shape and gene expression of cultured astrocytes.Restor Neurol Neurosci. 2019;37(4):291-313. doi: 10.3233/RNN-190903. Restor Neurol Neurosci. 2019. PMID: 31227672
-
Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice.Brain. 2009 Jun;132(Pt 6):1449-62. doi: 10.1093/brain/awp128. Epub 2009 May 19. Brain. 2009. PMID: 19454531
-
Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair.Neuropharmacology. 2014 Apr;79:456-66. doi: 10.1016/j.neuropharm.2013.09.014. Epub 2013 Sep 22. Neuropharmacology. 2014. PMID: 24067923 Free PMC article.
-
Schwann Cell Role in Selectivity of Nerve Regeneration.Cells. 2020 Sep 20;9(9):2131. doi: 10.3390/cells9092131. Cells. 2020. PMID: 32962230 Free PMC article. Review.
-
Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves.J Neurocytol. 1994 Jan;23(1):1-28. doi: 10.1007/BF01189813. J Neurocytol. 1994. PMID: 8176415 Review.
Cited by
-
The polysialic acid mimetics 5-nonyloxytryptamine and vinorelbine facilitate nervous system repair.Sci Rep. 2016 Jun 21;6:26927. doi: 10.1038/srep26927. Sci Rep. 2016. PMID: 27324620 Free PMC article.
-
6-Hydroxydopamine Induces Neurodegeneration in Terminally Differentiated SH-SY5Y Neuroblastoma Cells via Enrichment of the Nucleosomal Degradation Pathway: a Global Proteomics Approach.J Mol Neurosci. 2022 May;72(5):1026-1046. doi: 10.1007/s12031-021-01962-z. Epub 2022 Mar 8. J Mol Neurosci. 2022. PMID: 35258800 Free PMC article.
-
Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows.Biology (Basel). 2022 Dec 20;12(1):5. doi: 10.3390/biology12010005. Biology (Basel). 2022. PMID: 36671698 Free PMC article.
-
Nuclear fragments of the neural cell adhesion molecule NCAM with or without polysialic acid differentially regulate gene expression.Sci Rep. 2017 Oct 19;7(1):13631. doi: 10.1038/s41598-017-14056-x. Sci Rep. 2017. PMID: 29051583 Free PMC article.
-
Polysialic Acid Modulates the Binding of External Lactoferrin in Neutrophil Extracellular Traps.Biology (Basel). 2019 Mar 28;8(2):20. doi: 10.3390/biology8020020. Biology (Basel). 2019. PMID: 30925725 Free PMC article.
References
-
- Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. 2007;208:137–144. - PubMed
-
- Asahara T, Lin M, Kumazawa Y, Takeo K, Akamine T, Nishimura Y, Kayahara T, Yamamoto T. Long-term observation on the changes of somatotopy in the facial nucleus after nerve suture in the cat: morphological studies using retrograde labeling. Brain Res Bull. 1999;49:195–202. - PubMed
-
- Bolton SJ, Perry VH. Histone H1; a neuronal protein that binds bacterial lipopolysaccharide. J Neurocytol. 1997;26:823–831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
