Hepatocyte growth factor-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons
- PMID: 20844136
- PMCID: PMC6633456
- DOI: 10.1523/JNEUROSCI.3135-10.2010
Hepatocyte growth factor-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons
Abstract
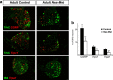
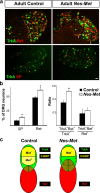
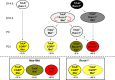
Nociceptors in peripheral ganglia display a remarkable functional heterogeneity. They can be divided into the following two major classes: peptidergic and nonpeptidergic neurons. Although RUNX1 has been shown to play a pivotal role in the specification of nonpeptidergic neurons, the mechanisms driving peptidergic differentiation remain elusive. Here, we show that hepatocyte growth factor (HGF)-Met signaling acts synergistically with nerve growth factor-tyrosine kinase receptor A to promote peptidergic identity in a subset of prospective nociceptors. We provide in vivo evidence that a population of peptidergic neurons, derived from the RUNX1 lineage, require Met activity for the proper extinction of Runx1 and optimal activation of CGRP (calcitonin gene-related peptide). Moreover, we show that RUNX1 in turn represses Met expression in nonpeptidergic neurons, revealing a bidirectional cross talk between Met and RUNX1. Together, our novel findings support a model in which peptidergic versus nonpeptidergic specification depends on a balance between HGF-Met signaling and Runx1 extinction/maintenance.
Figures





References
-
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. - PubMed
-
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. - PubMed
-
- Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–524. - PubMed
-
- Funakoshi H, Nakamura T. Identification of HGF-like protein as a novel neurotrophic factor for avian dorsal root ganglion sensory neurons. Biochem Biophys Res Commun. 2001;283:606–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
