Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear
- PMID: 20844149
- PMCID: PMC2963157
- DOI: 10.1523/JNEUROSCI.3042-10.2010
Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear
Abstract
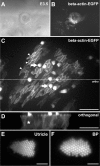
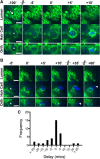
Epithelial homeostasis is essential for sensory transduction in the auditory and vestibular organs of the inner ear, but how it is maintained during trauma is poorly understood. To examine potential repair mechanisms, we expressed β-actin-enhanced green fluorescent protein (EGFP) in the chick inner ear and used live-cell imaging to study how sensory epithelia responded during aminoglycoside-induced hair cell trauma. We found that glial-like supporting cells used two independent mechanisms to rapidly eliminate dying hair cells. Supporting cells assembled an actin cable at the luminal surface that extended around the pericuticular junction and constricted to excise the stereocilia bundle and cuticular plate from the hair cell soma. Hair bundle excision could occur within 3 min of actin-cable formation. After bundle excision, typically with a delay of up to 2-3 h, supporting cells engulfed and phagocytosed the remaining bundle-less hair cell. Dual-channel recordings with β-actin-EGFP and vital dyes revealed phagocytosis was concurrent with loss of hair cell integrity. We conclude that supporting cells repaired the epithelial barrier before hair cell plasmalemmal integrity was lost and that supporting cell activity was closely linked to hair cell death. Treatment with the Rho-kinase inhibitor Y-27632 did not prevent bundle excision but prolonged phagocytic engulfment and resulted in hair cell corpses accumulating within the epithelium. Our data show that supporting cells not only maintain epithelial integrity during trauma but suggest they may also be an integral part of the hair cell death process itself.
Figures
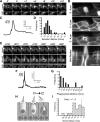

 p < 0.001). D, Phagosomes were also detected in P19 utricles after systemic administration of streptomycin in vivo. E, F, Phagosomes colocalize with phagocytic, but not macrophages markers. Phagosomes (arrowheads) and a macrophage (star) both label with anti-RAC1 (CED-10) (E). However, phagosomes do not label with anti-CD45 (F, bottom), unlike macrophages (F, top). Scale bars: 10 μm. Data are expressed as mean ± SEM.
p < 0.001). D, Phagosomes were also detected in P19 utricles after systemic administration of streptomycin in vivo. E, F, Phagosomes colocalize with phagocytic, but not macrophages markers. Phagosomes (arrowheads) and a macrophage (star) both label with anti-RAC1 (CED-10) (E). However, phagosomes do not label with anti-CD45 (F, bottom), unlike macrophages (F, top). Scale bars: 10 μm. Data are expressed as mean ± SEM.
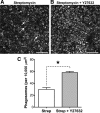
 p < 0.001). Data from Figure 4 were used as controls for statistical tests in C. Data are expressed as mean ± SEM. Scale bars: 50 μm.
p < 0.001). Data from Figure 4 were used as controls for statistical tests in C. Data are expressed as mean ± SEM. Scale bars: 50 μm.References
-
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006;218:20–29. - PubMed
-
- Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. - PubMed
-
- Bhave SA, Oesterle EC, Coltrera MD. Macrophage and microglia-like cells in the avian inner ear. J Comp Neurol. 1998;398:241–256. - PubMed
-
- Bohne BA, Rabbitt KD. Holes in the reticular lamina after noise exposure: implication for continuing damage in the organ of Corti. Hear Res. 1983;11:41–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
