Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation
- PMID: 20844192
- PMCID: PMC2953643
- DOI: 10.4049/jimmunol.1000715
Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation
Abstract
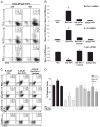
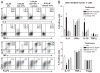
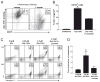
Galectin-1 (Gal-1), a β-galactoside-binding lectin, plays a profound role in modulating adaptive immune responses by altering the phenotype and fate of T cells. Experimental data showing recombinant Gal-1 (rGal-1) efficacy on T cell viability and cytokine production, nevertheless, is controversial due to the necessity of using stabilizing chemicals to help retain Gal-1 structure and function. To address this drawback, we developed a mouse Gal-1 human Ig chimera (Gal-1hFc) that did not need chemical stabilization for Gal-1 ligand recognition, apoptosis induction, and cytokine modulation in a variety of leukocyte models. At high concentrations, Gal-1hFc induced apoptosis in Gal-1 ligand(+) Th1 and Th17 cells, leukemic cells, and granulocytes from synovial fluids of patients with rheumatoid arthritis. Importantly, at low, more physiologic concentrations, Gal-1hFc retained its homodimeric form without losing functionality. Not only did Gal-1hFc-binding trigger IL-10 and Th2 cytokine expression in activated T cells, but members of the CD28 family and several other immunomodulatory molecules were upregulated. In a mouse model of contact hypersensitivity, we found that a non-Fc receptor-binding isoform of Gal-1hFc, Gal-1hFc2, alleviated T cell-dependent inflammation by increasing IL-4(+), IL-10(+), TGF-β(+), and CD25(high)/FoxP3(+) T cells, and by decreasing IFN-γ(+) and IL-17(+) T cells. Moreover, in human skin-resident T cell cultures, Gal-1hFc diminished IL-17(+) T cells and increased IL-4(+) and IL-10(+) T cells. Gal-1hFc will not only be a useful new tool for investigating the role of Gal-1 ligands in leukocyte death and cytokine stimulation, but for studying how Gal-1-Gal-1 ligand binding shapes the intensity of immune responses.
Figures






Similar articles
-
Galectin-1 research in T cell immunity: past, present and future.Clin Immunol. 2012 Feb;142(2):107-16. doi: 10.1016/j.clim.2011.09.011. Epub 2011 Oct 6. Clin Immunol. 2012. PMID: 22019770 Free PMC article. Review.
-
Transgenic galectin-1 induces maturation of dendritic cells that elicit contrasting responses in naive and activated T cells.J Immunol. 2006 Jun 15;176(12):7207-20. doi: 10.4049/jimmunol.176.12.7207. J Immunol. 2006. PMID: 16751364
-
Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion.J Immunol. 2008 Mar 1;180(5):3091-102. doi: 10.4049/jimmunol.180.5.3091. J Immunol. 2008. PMID: 18292532
-
Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis.J Exp Med. 1999 Aug 2;190(3):385-98. doi: 10.1084/jem.190.3.385. J Exp Med. 1999. PMID: 10430627 Free PMC article.
-
Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments.Immunol Rev. 2009 Jul;230(1):144-59. doi: 10.1111/j.1600-065X.2009.00787.x. Immunol Rev. 2009. PMID: 19594634 Review.
Cited by
-
The Role of Galectin-9 as Mediator of Atopic Dermatitis: Effect on Keratinocytes.Cells. 2021 Apr 20;10(4):947. doi: 10.3390/cells10040947. Cells. 2021. PMID: 33923930 Free PMC article.
-
Galectin-1 research in T cell immunity: past, present and future.Clin Immunol. 2012 Feb;142(2):107-16. doi: 10.1016/j.clim.2011.09.011. Epub 2011 Oct 6. Clin Immunol. 2012. PMID: 22019770 Free PMC article. Review.
-
The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with galectin-1.Mol Cell Biol. 2014 Jul;34(13):2479-87. doi: 10.1128/MCB.00348-14. Epub 2014 Apr 21. Mol Cell Biol. 2014. PMID: 24752896 Free PMC article.
-
Engineering galectin-glycan interactions for immunotherapy and immunomodulation.Exp Biol Med (Maywood). 2016 May;241(10):1074-83. doi: 10.1177/1535370216650055. Exp Biol Med (Maywood). 2016. PMID: 27229902 Free PMC article. Review.
-
Cell-surface receptor control that depends on the size of a synthetic equilateral-triangular RNA-protein complex.Sci Rep. 2014 Sep 19;4:6422. doi: 10.1038/srep06422. Sci Rep. 2014. PMID: 25234354 Free PMC article.
References
-
- Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. - PubMed
-
- Garín MI, Chu CC, Golshayan D, Cernuda-Morollón E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+ CD25+ T cells. Blood. 2007;109:2058–2065. - PubMed
-
- Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

